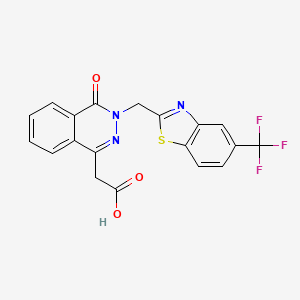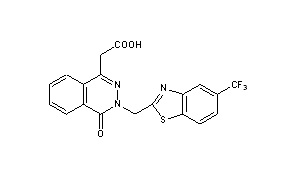
Zopolrestat
CAS : 110703-94-1
110765-49-6 (Na salt)
3,4-Dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl]methyl]-1-phthalazineacetic acid
2- [4-Oxo-3- [5- (trifluoromethyl) benzothiazol-2-ylmethyl] -3,4-dihydrophthalazin-1-yl] acetic acid
3-(5-trifluoromethylbenzothiazol-2-ylmethyl)-4-oxo-3H-phthalazin-1-ylacetate
Pfizer Inc. INNOVATOR
2-[4-oxo-3-[5-(trifluoromethyl)benzothiazol-2-ylmethyl]-3,4-dihydrophthalazin-1-yl]acetic acid
Manufacturers’ Codes: CP-73850
MF: C19H12F3N3O3S
MW: 419.38
C 54.41%, H 2.88%, F 13.59%, N 10.02%, O 11.45%, S 7.65%
Crystals, mp 197-198°. pKa (dioxane/water): 5.46 (1:1); 6.38 (2:1). Log P (n-octanol/water): 3.43.
mp 197-198°
pKa: pKa (dioxane/water): 5.46 (1:1); 6.38 (2:1)
Log P: Log P (n-octanol/water): 3.43
Therap-Cat: Treatment of diabetic complications.
Keywords: Aldose Reductase Inhibitor.
…………………………..
synthesis
2-(8-oxo-7-((5-trifluromethyl)-1H-benzo[d]imidazol-2-yl)methyl)7,8-dihydropyrazin[2,3-d]pyridazin-5-yl)acetic acid and [4-oxo-(5-trifluoromethyl-benzothaiazol-2-ylmethyl)-3,4-dihydro-phthalazin-1-yl]-acetic acid (also known as zopolrestat), pharmaceutical compositions thereof and methods of treating diabetic complications in mammals comprising administering to mammals these salt and compositions. 2-(8-oxo-7-((5-trifluromethyl)-1H-benzo[d]imidazol-2-yl)methyl)8-dihydropyrazin[2,3-d]pyridazin-5-yl) acetic acid (formula II), is disclosed in WO 2012/009553 A1. Zopolrestat (formula III) is disclosed in U.S. Pat. No. 4,939,140.
Each of the patents, applications, and other references referred to herein are incorporated by reference. The diabetic complications include neuropathy, nephropathy, retinopathy, cataracts and cardiovascular complications, including myocardial infarction and cardiomyopathy. This invention is also directed to combinations of these salts and antihypertensive agents. These combinations are also useful in treating diabetic complications in mammals.
2-(8-oxo-7-((5-trifluoromethyl)-1H-benzo[d]imidazol-2-yl)methyl)8-dihydropyrazin[2,3-d]pyridazin-5-yl)acetic acid is prepared as disclosed in WO 2012/009553 A1, which is incorporated herein by reference. Zopolrestat is prepared as disclosed in U.S. Pat. No. 4,939,140.
…………………………

Zopolrestat can be obtained by several different ways: 1) The reaction of 2- (4-oxo-3,4-dihydrophthalazin-1-yl) acetic acid ethyl ester (I) with 2-chloroacetonitrile by means of potassium tert-butoxide in DMF gives 2- [3- (cyanomethyl) -4-oxo-3,4-dihydrophthalazin-1-yl] acetic acid ethyl ester (II), which is cyclized with 2-amino-4- (trifluoromethyl) thiophenol (III) in refluxing ethanol yielding zopolrestat ethyl ester (IV). Finally, this compound is hydrolyzed with KOH in methanol / water / THF. 2) Compound (IV) can also be obtained by cyclization of (II) with 4-chloro-3-nitrobenzotrifluoride . (V) in hot DMF saturated with H2S 3) Compound (II) can also be obtained as follows: The reaction of phthalazine (I) with aqueous formaldehyde gives 2- [3- (hydroxymethyl) -4-oxo-3,4 -dihydrophthalazin-1-yl] acetic acid ethyl ester (VI), which is treated with PBr3 in ethyl ether yielding the bromomethyl derivative (VII). Finally, this compound is treated with potassium cyanide and KI in acetone / water.


……………………….
5=CF3 IS SUBS
……………………..
EXAMPLE 18 Sodium 3-(5-trifluoromethylbenzothiazol-2-ylmethyl)-4-oxo-3H-phthalazin-1-ylacetateSodium methoxide (54 mg) was added to 3-(5-trifluoromethylbenzothiazol-2-ylmethyl)-4-oxo-phthalazin-1-ylacetic acid (0.4 g) in methanol 10 ml) at room temperature. After the addition was complete, a clear solution was obtained which was stirred for 15 minutes at room temperature. The excess methanol was evaporated. The residue was triturated with ether (20 ml) and filtered to obtain the product (0.43 g; m.p. 300° C.).EXAMPLE 19 3-(5-Trifluoromethylbenzothiazol-2-ylmethyl)-4-oxo-3H-phthalazin-1-ylacetate, dicyclohexylamine saltTo a mixture of 3-(5-trifluromethylbenzothiazol-2ylmethyl)-4-oxo-phthalazin-1-ylacetic acid (0.42 g) in methanol (10 ml) was added dicyclohexylamine (0.2 g) in methanol (5 ml). The resulting clear solution was stirred at room temperature for 15 minutes and then evaporated to dryness. Trituration of the residue with ether (30 ml) gave a white solid (0.38 g; m.p. 207° C.).EXAMPLE 20 3-(5-Trifluoromethylbenzothiazol-2ylmethyl)-4-oxo-3H-phthalazin-1-ylacetic acid, meglumine saltA solution of 3-(5-trifluoromethylbenzothiazol-2-ylmethyl)-4-oxo-phthalazin-1-ylacetic acid (419 mg) and meglumine (196 mg) in methanol (50 ml) was stirred at room temperature for an hour and then evaporated to dryness. The residue was triturated with ether (25 ml), filtered and the collected solid was air dried (610 mg; m.p. 157° C.)……………………………
J. Med. Chem., 1991, 34 (1), pp 108–122
DOI: 10.1021/jm00105a018
http://pubs.acs.org/doi/abs/10.1021/jm00105a018
……………………………………
Mylari, Banavara L.; Zembrowski, William J.; Beyer, Thomas A.; Aldinger, Charles E.; Siegel, Todd W.
Journal of Medicinal Chemistry, 1992 , vol. 35, 12 p. 2155 – 2162
………………………………..
Mylari; Beyer; Scott; Aldinger; Dee; Siegel; Zembrowski
Journal of Medicinal Chemistry, 1992 , vol. 35, 3 p. 457 – 465
…………………………….
Literature References:
Aldose reductase inhibitor. Prepn: B. L. Mylari et al., EP 222576; E. R. Larson, B. L. Mylari, US 4939140(1987, 1990 both to Pfizer);
B. L. Mylari et al. J. Med. Chem. 34, 108 (1991).
Pharmacology: B. Tesfamariam et al., J. Cardiovasc.Pharmacol. 21, 205 (1993); B. Tesfamariam et al., Am. J. Physiol. 265, H1189 (1993).
Clinical pharmacokinetics: P. B. Inskeep et al., J. Clin. Pharmacol. 34, 760 (1994).
Zopolrestat < Rec INN; BAN; USAN >
Drugs Fut 1995, 20(1): 33
Drugs Fut 1995, 20(1): 33
Synthesis of aldose reductase inhibitor, 3, 4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2 14C benzothiazolyl]methyl]-1-phthalazineacetic acid
J Label Compd Radiopharm 1991, 29(2): 143
J Label Compd Radiopharm 1991, 29(2): 143
|
3-19-1992
|
HETEROCYCLIC OXOPHTHALAZINYL ACETIC ACIDS
|
|
|
3-6-1992
|
3-(5-TRIFLUOROMETHYLBENZOTHIAZOL-2-YLMETHYL)-4-OXO-3H-PHYTHALAZIN-1-YLACETIC ACID MONOHYDRATE
|
|
|
7-4-1990
|
Heterocyclic oxophthalazinyl acetic acids
|
|
3-24-2006
|
Medical devices to treat or inhibit restenosis
|
|
|
12-30-2004
|
N-[(SUBSTITUTED FIVE-MEMBERED DI- OR TRIAZA DIUNSATURATED RING)CARBONYL]GUANIDINE DERIVATIVES FOR THE TREATMENT OF ISCHEMIA
|
|
|
10-7-2004
|
COMBINATION OF AN ALDOSE REDUCTASE INHIBITOR AND A GLYCOGEN PHOSPHORYLASE INHIBITOR COMBINATION OF AN ALDOSE REDUCTASE INHIBITOR AND A GLYCOGEN PHOSPHORYLASE INHIBITOR
|
|
|
9-30-2004
|
Aldose reductase inhibition in preventing or reversing diabetic cardiomyopathy
|
|
|
5-27-2004
|
SUBSTITUTED FUSED HETEROCYCLIC COMPOUNDS
|
|
|
4-15-2004
|
Compounds for treating and preventing diabetic complications
|
|
|
3-32-2004
|
IMPROVED MUTANTS OF (2,5-DKG) REDUCTASE A
|
|
|
12-18-2003
|
Pharmaceutical composition for use in treatment of diabetes
|
|
|
11-14-2003
|
Salts of zopolrestat
|
|
|
4-18-2002
|
Use of an aldose reductase inhibitor for reducing non-cardiac tissue damage
|