SIMPONI® Receives European Commission Approval for Treatment of Moderately to Severely Active Ulcerative Colitis

SIMPONI® , golimumab
First and Only Subcutaneous Biologic Treatment Administered Every Four Weeks Approved for Ulcerative Colitis
LEIDEN, The Netherlands, Sept. 23, 2013 /PRNewswire/ — Janssen Biologics B.V. (“Janssen”) announced today that the European Commission has approved SIMPONI® (golimumab) for the treatment of moderately to severely active ulcerative colitis (UC) in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies. The European Commission approval follows a positive opinion by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) in July 2013 recommending the use of SIMPONI.
read all at
http://www.pharmalive.com/eu-oks-simponi-for-ulcerative-colitis
Golimumab (Simponi; Centocor Ortho Biotech), a fully human antibody that is specific for tumour necrosis factor, was approved by the US FDA for the treatment of rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis in April 2009.

Golimumab
In the past decade, the introduction of biologics that inhibit the activity of the pro-inflammatory cytokine tumour necrosis factor (TNF) has revolutionized the treatment of a range of immuno-inflammatory disorders, such as rheumatoid arthritis, psoriasis and Crohn’s disease1. The first two such biologics — infliximab (Remicade; Centocor/Schering-Plough), a chimeric monoclonal antibody (mAb) specific for TNF, and etanercept (Enbrel; Amgen/Wyeth), a fusion protein that contains the ligand-binding portion of the soluble TNF receptor — were approved for the treatment of rheumatoid arthritis in the late 1990s. Their use has since been expanded to other disorders, including psoriatic arthritis. In 2002, the fully human TNF-specific mAb adalimumab (Humira; Abbott) was approved for the treatment of rheumatoid arthritis and is now also approved for several other immuno-inflammatory disorders. A fourth TNF inhibitor, the PEGylated humanized TNF-specific antibody fragment certolizumab pegol (Cimzia; UCB), was approved for Crohn’s disease in 2008 and rheumatoid arthritis in May 2009.
Golimumab, in combination with MTX, is approved by the FDA for the treatment of adult patients with moderately to severely active rheumatoid arthritis. It is also approved for the treatment of adult patients with active psoriatic arthritis (alone or in combination with MTX) and for the treatment of adult patients with active ankylosing spondylitis



ZOLMITRIPTAN
A paper from Emcure
Four isomeric unknown impurities ranging from 0.08-0.12% were found in the purified sample of Zolmitriptan during the batch analysis by gradient reverse phase ultra performance liquid chromatography (UPLC) and their molecular weights determined by liquid chromatography mass spectroscopy (LC-MS) analysis. Subsequently, all the four impurities were isolated by flash chromatography followed by semi-preparative HPLC and characterized by 1H NMR, 13C NMR, 1H-1H COSY, HMBC, HSQC, MS spectroscopy and HPLC. The structures for these four impurities were assigned to be following
Isomeric Impurity-1: 4-((3-(2-(dimethylamino)ethyl)-4-(2-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one,
Isomeric Impurity-2: 4-((3-(2-(dimethylamino)ethyl)-2-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one-,
Isomeric Impurity-3: 4-((3-(2-(dimethylamino)ethyl)-7-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one,
Isomeric Impurity-4: 4-((3-(2-(dimethylamino)ethyl)-6-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one
Isolation and characterization of impurities has helped us in improving the purity of API by removing these impurities using crystallization.
READ ALL THIS AT
Neelakandan K
API Research Centre
Emcure Pharmaceutical Limited
Hinjawadi, Pune, 411057, India
Fax: +91 20 39821445
E-mail: Neelakandan.K@emcure.co.in

| Volume 4, Issue 2 | |||||
|
………….
Mukund Keshao Gurjar
Dr. Mukund Gurjar is an Executive Director and Chief Scientific Officer (Research and Development) of this Company(Emcure). He is a graduate, a post graduate and Ph.D. in Chemistry from the Nagpur University. He also holds a second Ph. D. degree in Chemistry from the London University, United Kingdom as well as a post doctoral fellowship from Toronto, Canada. Prior to joining our Company, he was the deputy director of the National Chemical Laboratory, Pune where he spent 25 years spearheading innovative and advance research in Organic Chemistry. He has over 32 years of experience in pharmaceutical sciences and is a fellow at various national and international academies. He is a member of the editorial board of the prestigious journal Organic Process Research & Development published by the American Chemical Society. For his contributions to synthetic organic chemistry involving both basic and applied research, he has been felicitated with various awards. A large number of students have obtained Ph.Ds under the supervision of Dr. Gurjar and has published more than 200 papers in various international journals. He has been associated with our Company since 2001 and also became a member of the Board in the same year.
Takeda Gets Simultaneous EU OKs for Three Type 2 Diabetes Therapies
Takeda Receives Simultaneous European Marketing Authorization for Three New Type 2 Diabetes Therapies, VipidiaTM (alogliptin) and Fixed-Dose Combinations VipdometTM (alogliptin and metformin) and IncresyncTM (alogliptin and pioglitazone)
Osaka, Japan, September 24, 2013 – Takeda Pharmaceutical Company Limited (Takeda) today announced that the European Commission has granted Marketing Authorization (MA) for VipidiaTM (alogliptin), a dipeptidyl peptidase IV (DPP-4) inhibitor, for the treatment of type 2 diabetes patients who are uncontrolled on existing therapies1-3and for the fixed-dose combination (FDC) therapies VipdometTM (alogliptin with metformin) and IncresyncTM (alogliptin with pioglitazone). The Committee for Medicinal Products for Human Use (CHMP), of the European Medicines Agency (EMA), issued a positive opinion for these products on July 26, 2013.http://www.pharmalive.com/takeda-gets-simultaneous-eu-oks-for-three-new-type-2-diabetes-therapies
Health Canada Approves Bayer’s Hypertension Drug
riociguat
Bayer Inc. announced today that the Health Canada has approved the drug Adempas (riociguat) for the treatment of inoperable, or persistent and recurrent chronic thromboembolic pulmonary hypertension (CTEPH) after surgery in adult patients. Learn more…http://www.dddmag.com/news/2013/09/health-canada-approves-bayers-hypertension-drug?et_cid=3497158&et_rid=523035093&type=headline
Roche Gets Breakthrough Status for Lung Cancer Drug
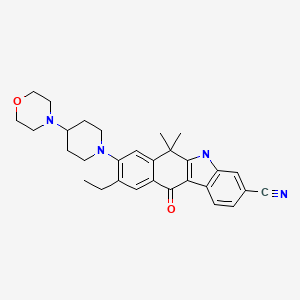
ALECTINIB
http://www.who.int/medicines/publications/druginformation/issues/PL_108.pdf
9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-
6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
tyrosine kinase inhibitor, antineoplastic
C30H34N4O2, CAS 1256580-46-7
The U.S. Food and Drug Administration (FDA) has granted breakthrough therapy designation for Roche’s alectinib – a promising investigational 2nd generation ALK inhibitor – based on data that will be presented at European Cancer Congress (ECC). Read more…http://www.dddmag.com/news/2013/09/roche-gets-breakthrough-status-lung-cancer-drug?et_cid=3497158&et_rid=523035093&type=cta

Teva Launches Generic Niaspan in U.S.

niaspan
Teva Announces Exclusive Launch of Generic NIASPAN® in the United States
JERUSALEM–(BUSINESS WIRE)–Teva Pharmaceutical Industries Ltd. (NYSE:TEVA) today announced the launch of the generic equivalent to NIASPAN® (niacin extended-release) tablets, 500, 700, and 1000mg in the United States. Teva was first to file, making the product eligible for 180 days of marketing exclusivity.
NIASPAN® is marketed by AbbVie and used with diet to reduce elevated TC, LDL-C, Apo B and TG levels, and to increase HDL-C in patients with primary hyperlipidemia and mixed dyslipidemia. NIASPAN® had annual sales of approximately $1.12 billion in the United States, according to IMS data as of June 30, 2013. read more at
http://www.pharmalive.com/teva-launches-generic-niaspan-in-us


niacin, vit B3
Niacin (also known as vitamin B3, nicotinic acid, or less commonly vitamin PP; archaic terms include pellagra-preventive and anti-dermatitis factor) is an organic compound with the formula C
6H
5NO
2 and, depending on the definition used, one of the 40 to 80 essential human nutrients.
Niacin is one of five vitamins (when lacking in human diet) associated with a pandemic deficiency disease: niacin deficiency (pellagra), vitamin C deficiency (scurvy), thiamin deficiency (beriberi), vitamin D deficiency (rickets and osteomalacia), vitamin A deficiency (night blindness and other symptoms). Niacin has been used for over 50 years to increase levels of HDL in the blood and has been found to decrease the risk of cardiovascular events modestly in a number of controlled human trials.[3]
This colorless, water-soluble solid is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position. Other forms of vitamin B3 include the corresponding amide, nicotinamide (“niacinamide”), where the carboxyl group has been replaced by a carboxamide group (CONH
2), as well as more complex amides and a variety of esters. Nicotinic acid and niacinamide are convertible to each other with steady world demand rising from 8500 tonnes per year in 1980s to 40,000 in recent years.[4]
Niacin cannot be directly converted to nicotinamide, but both compounds could be converted to and are precursors of NAD andNADP in vivo.[5] Nicotinic acid, nicotinamide, and tryptophan (via quinoline acid) are co-factors for nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). NAD converts to NADP by phosphorylation in the presence of the enzyme NAD+ kinase. NADP and NAD are coenzyme for many dehydrogenases, participating in many hydrogen transfer processes.[6] NAD is important in catabolism of fat, carbohydrate, protein, and alcohol, as well as cell signaling and DNA repair, and NADP mostly in anabolism reactions such as fatty acid and cholesterol synthesis.[6] High energy requirements (brain) or high turnover rate (gut, skin) organs are usually the most susceptible to their deficiency.[7]
Although the two are identical in their vitamin activity, nicotinamide does not have the same pharmacological effects (lipid modifying effects) as niacin. Nicotinamide does not reduce cholesterol or cause flushing.[8] Nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[9] Niacin is involved in both DNA repair, and the production of steroid hormones in theadrenal gland.
- “Niacin”. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Retrieved 14-January-2012.
- PubChem 938
- Bruckert, E; Labreuche, J; Amarenco, P (2010 Jun). “Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis”.Atherosclerosis 210 (2): 353–61. doi:10.1016/j.atherosclerosis.2009.12.023.PMID 20079494.
- Cantarella, L; Gallifuoco, A; Malandra, A; Martínková, L; Spera, A; Cantarella, M (2011). “High-yield continuous production of nicotinic acid via nitrile hydratase-amidase cascade reactions using cascade CSMRs”. Enzyme and microbial technology 48 (4–5): 345–50. doi:10.1016/j.enzmictec.2010.12.010. PMID 22112948.
- Cox, Michael; Lehninger, Albert L; Nelson, David R. (2000). Lehninger principles of biochemistry. New York: Worth Publishers. ISBN 1-57259-153-6.
- ^ Jump up to:a b Wan, P; Moat, S; Anstey, A (2011). “Pellagra: A review with emphasis on photosensitivity”. The British journal of dermatology 164 (6): 1188–200.doi:10.1111/j.1365-2133.2010.10163.x. PMID 21128910.
- Ishii, N; Nishihara, Y (1981). “Pellagra among chronic alcoholics: Clinical and pathological study of 20 necropsy cases”. Journal of neurology, neurosurgery, and psychiatry 44 (3): 209–15. doi:10.1136/jnnp.44.3.209. PMC 490893.PMID 7229643.
- Jaconello P (October 1992). “Niacin versus niacinamide”. CMAJ 147 (7): 990.PMC 1336277. PMID 1393911.
- Knip M, Douek IF, Moore WP, et al. (2000). “Safety of high-dose nicotinamide: a review”. Diabetologia 43 (11): 1337–45. doi:10.1007/s001250051536.PMID 11126400.
Food sources
“Food Data Chart – Niacin”. Retrieved 7 September 2012.
Niacin is found in variety of foods, including liver, chicken, beef, fish, cereal, peanuts and legumes, and is also synthesized from tryptophan, an essential amino acid found in most forms of protein.
Animal products:
- liver, heart and kidney (9 – 15 mg niacin per 100 grams)
- chicken, chicken breast (6.5 mg)
- beef (5 – 6 mg)
- fish: tuna, salmon, halibut (2.5 – 13 mg)
- eggs (0.1 mg)
- venison (8.43 mg)
Fruits and vegetables:
- avocados (1 mg niacin per 100 grams)
- dates (2 mg)
- tomatoes (0.7 mg)
- leaf vegetables (0.3 – 0.4 mg)
- broccoli (0.6 mg)
- carrots (0.3 – 0.6 mg)
- sweet potatoes (0.5 – 0.6 mg)
- asparagus (0.4 mg)
Seeds:
- nuts (2 mg niacin per 100 grams)
- whole grain products (4 – 29.5 mg)
- legumes (0.4 – 16 mg)
- saltbush seeds
Fungi:
- mushrooms, shiitake mushrooms (3.5 – 4 mg niacin per 100 grams)
- brewer’s yeast (36 mg)
Other:
- Monster Energy drink (40 mg per 16 ounces)
- Rockstar Energy (100% in the Super Sours flavors)
- Red Bull Energy Drink (28 mg per 12 ounces)
- Five Hour Energy drink (30 mg per 1.93 ounces)
- Ovaltine (18 mg)
- Peanut butter (15 mg)
- Tofu
- Soy sauce (0.4 mg)
- Vegemite (from spent brewer’s yeast) (110 mg niacin per 100 grams)
- Marmite (from spent brewer’s yeast) (110 mg niacin per 100 grams)













 Melting corks allow for temperature-controlled release of drugs from microscale vessels
Melting corks allow for temperature-controlled release of drugs from microscale vessels A novel nanoparticle-based drug delivery system prevents the premature release of therapeutic compounds
A novel nanoparticle-based drug delivery system prevents the premature release of therapeutic compounds