
ALLOW SLIDESHARE TO LOAD…………
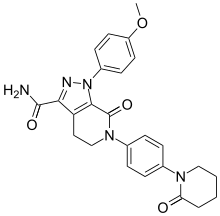
APIXABAN
Bristol-Myers Squibb and Pfizer announced that the U.S. Food and Drug Administration has accepted for review a Supplemental New Drug Application for Eliquis, for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism, in patients who have undergone hip or knee replacement surgery.FULL STORY
Apixaban (INN, trade name Eliquis) is an anticoagulant for the prevention of venous thromboembolism and venous thromboembolic events. It is a direct factor Xa inhibitor. Apixaban has been available in Europe since May 2011 and was approved for preventing venous thromboembolism after elective hip or knee replacement. The FDA approved apixaban in December 2012 with an indication of reducing the risk of stroke and dangerous blood clots (systemic embolism) in patients with atrial fibrillation that is not caused by a heart valve problem. The drug was developed in a joint venture by Pfizer and Bristol-Myers Squibb

The challenge of delivering drugs to neurological targets has been a major roadblock to many promising therapies for treating diseases and disorders of the brain. The blood brain barrier blocks the vast majority of all small-molecules and virtually all large molecules from reaching therapeutic targets within the brain.FULL STORY
Comparative Study of Dossier Compilation & Submission Process of Drug Product in USA, Europe & India.
wait for slideshare to load…………….

Amyvid
Eli Lilly & Co. says it will push ahead with an imaging chemical designed to help screen for Alzheimer’s disease, despite a negative ruling by Medicare officials. The Centers for Medicare and Medicaid Services said last week it will not cover the chemical which highlights brain plaque in medical imaging scans.
Eli Lilly & Co. says it will push ahead with a first-of-a-kind imaging chemical designed to help screen for Alzheimer’s disease, despite a negative ruling by Medicare officials.
The Centers for Medicare and Medicaid Services said last week it will not cover the chemical, called Amyvid, which highlights brain plaque in medical imaging scans. The government program provides health coverage to more than 47 million seniors, and is the largest payer for prescription drugs in the U.S.
Amyvid contains florbetapir F 18, a molecular imaging agent that binds to β-amyloid aggregates, and is intended for use with PET imaging of the brain. Chemically, florbetapir F 18 is described as (E)-4-(2-(6-(2-(2-(2[18F] fluoroethoxy)ethoxy)ethoxy)pyridine3-yl)vinyl)-N-methylbenzamine. The molecular weight is 359 and the structural formula is:
 |
Amyvid is a sterile, non-pyrogenic radioactive diagnostic agent for intravenous injection. The clear, colorless solution is supplied ready to use and each milliliter contains 0.1 to 19 micrograms of florbetapir and 500 – 1900 MBq (13.5 – 51 mCi) florbetapir F 18 at EOS, with 4.5 mg sodium ascorbate USP, and 0.1 mL dehydrated alcohol USP in 0.9% sodium chloride injection USP. The pH of the solution is between 5.5 and 7.5.
Amyvid is radiolabeled with [18F] fluorine (F 18) that decays by positron (β+) emission to O 18 and has a half-life of 109.77 minutes. The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 3).
Table 3: Principal Radiation Produced from Decay of Fluorine 18
| Radiation | Energy Level (keV) | Abundance (%) |
| Positron | 249.8 | 96.9 |
| Gamma | 511 | 193.5 |
Nanotechnology can be defined as a technology which deals with manipulation, study, and designing and developing particles, bio-molecules of the size more than 1 nm and less than 100 nanometer, with the intention of modification enhancement or lowering a particular property of a molecule or a particle, which can be used in developing a device or molecule
.Nanotechnology involves developing materials or devices in the size range of 1 nm to 100 nanometer. At this scale quantum mechanical effects have very important implications in the quantum realm; nanotechnology controls the properties of material on an atomic level.

A serious cause of concern about nanotechnology is its safety and hazardous effects on environment and health, nanomaterial is required to be handled with special care and requires special methods for its disposal.
Drugs that use nanotechnology are also required to qualify for its effectiveness and safety, safety studies are very important factors as so far there is not enough data of drugs developed using nanotechnology and tested for safety.
Nanostructures provide this surface with superhydrophobicity, which lets water droplets roll down the inclined plane.
In pharmaceuticals nanotechnology has wide applications some of which are given below.
1. Targeting a drug to a particular tissue, to, enhancing absorption of a drug molecule in a particular tissue
2. To reduce degradation of a drug and enhance bioavailability and reduce untoward toxic effect of a drug molecule.
3. To enhance the microbial stability of a product
4. In cosmetics zinc oxide nanoparticles are used to increase its antimicrobial properties , and titanium dioxide nano particles effectively block UV rays in both cases concentrations required are very low compared to conventional use.
5. Nanoemulsions for increasing the absorption of a drug molecules.
6. To develop molecules as tracer marker compound to identify the toxic and untoward effects or spilage
Graphical representation of a rotaxane, useful as a molecular switch.


This DNA tetrahedron is an artificially designed nanostructure of the type made in the field of DNA nanotechnology. Each edge of the tetrahedron is a 20 base pair DNA double helix, and each vertex is a three-arm junction.
This device transfers energy from nano-thin layers of quantum wells to nanocrystals above them, causing the nanocrystals to emit visible light.
A team of scientists from the Massachusetts Institute of Technology and Brigham and Women’s Hospital conducted study. They stored an prodrug of cisplatin (which is used in most of cancer chemotherapies) within nanoparticles which they developed to target a specific protein in cancerous cells in prostate gland.
After these prodrug loaded nanoparticles were absorbed by cancerous cells the prodrug was released in to the cancerous cells and was converted in to an active form . The team demonstrated that these prodrug carrying nanoparticles were able to kill cancer cells in culture more efficiently than the drug alone.

Study was conducted by researchers, led by Dr. Omid Farokhzad and Dr. Stephen Lippard, to study nanoparticle drug delivery system for an effective and safer option for chemotherapy in living animals. Their research work is published in Proceedings of the National Academy of Sciences, in Jan 2011 issue of the journal, the study was funded in part by NIH’s National Cancer Institute (NCI) and National Institute for Biomedical Imaging and Bioengineering (NIBIB).
By applying this drug delivery by nanoparticles they were able to shrink tumors in mice with smaller doses of the drug to reduce harmful side effects. Only 30% of the dose of prodrug of cisplatin was required to diminish the tumor by using the drug carrying nanoparticles, than that of standard dose of cisplatin as such.
Researchers initially studied different doses of nanoparticle bound drug in rats and mice, both the types of animals maintained their body weight and survived at higher doses of the drug when drug was delivered using nanoparticles than when injected without nanoparticles. It was also found that the kidney damage was less in rats which received the nanoparticle bound drug.
Also it was found that binding nanoparticles provided greater stability of cisplatin prodrug in blood stream than that of injected alone , after one hour about 77 % of prodrug was found in blood stream when it was delivered using nanoparticles compared to only 16% available drug in case of drug delivered without nanoparticles, cispaltin is very unstable drug and remains in blood for very short time , which calls for more dose to get the desired effect.
US FDA issued new guidelines for Transdermal drug delivery system and related drug delivery systems.
US FDA stated in its new guidelines on transdermal drug delivery system and related drug delivery systems that the initial drug load concentration has tremendous potential for impacting quality of product its safety and efficacy and it has great potential for drug abuse.
There are many advantages and disadvantages of transdermal drug delivery system TDDS , like a drug can be administered without pain to patient, patients to like the dosage form greatly as they wont feel as they are on medication, and a constant plasma drug concentration can be easily achieved for a drug for a longer period of time without giving the untoward effect of initial higher plasma level of a drug as in case of conventional dosage forms, also drug escape the first pass metabolism through transdermal drug delivery , some a critical drugs which are known to save life are also administered as Transdermal patches for example nitroglycerin in congestive cardiac diseases.
There are some serious effects observed in resent time, like accidental high dose of a drug up on accidental sticking on handling or accidental contact with skin which has lead individual serious and to fatal conditions some times a life threatening one.
The fatal untoward effects are also seen in health care providers which accidentally handled the patches and got drug dose from remaining drug load from the used transdermal drug delivery patch.
The important factor.
The drug concentration which is required to be loaded on to a Transdermal drug delivery or related drug delivery systems is very high than that of the actual drug being absorbed and required to be achieved in to plasma of a patient.

Nanotechnology cancer treatments would use gold particles to carry anticancer drugs straight to the cancer. Learn about nanotechnology cancer treatments.
US FDA guidelines for Transdermal drug delivery patches and related drug delivery systems
In order to finally achieve consistent low residual drug with the desired quality of the Transdermal drug delivery systems ,
1. US FDA requires a drug manufacturers to submit the initial loaded drug concentration in the transdermal drug delivery patch and related drug delivery systems , be provided in the application for investigational new drug applications (INDs), new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplemental new drug applications (sNDAs) for TDDS, TMDS, and topical patch products.
2.US FDA now requires that the all justifications for initial drug load or concentration should be included in the application.
3.It also states that a proper scientific risk based approach must be taken to minimize the drug residue in the system so that a lowest possible concentration remains in the system.
4.The amount of residual drug in the transdemanl drug delivery system must not exceed than those already approved by FDA .
5. US FDA also requires that the information of product and process development and how the final formulation is justified should be given in the common technical document (CTD) formatted application in section for Pharmaceutical Development.
US FDA has put emphasis on following points
1.) Quality By Design Concept
2.) Minimizing Residual Drug
The transdermal drug delivery patches and related products , be developed with the intention of giving efficacy and safety as well,
The quality by design concept basically requires a formulator to plan for a desired quality, quality of a drug can be best achieved when it is planed than when it monitored.
Planing of quality of a drug product through logical application of past findings and research data and chemistry of drug molecule and exceipients being used, to achieve minimum drug load and this can lead to achieve minimum residual drug in transdermal drug delivery systems after use. Which will ensure that the abuse potential of the transdermal drug delivery systems are taken care of.
Buckminsterfullerene C60, also known as the buckyball, is a representative member of the carbon structures known as fullerenes. Members of the fullerene family are a major subject of research falling under the nanotechnology umbrella.
Nanotechnology is one of the most popular areas of scientific research, especially with regard to medical applications. We’ve already discussed some of the new detection methods that should bring about cheaper, faster and less invasive cancer diagnoses. But once the diagnosis occurs, there’s still the prospect of surgery, chemotherapy or radiation treatment to destroy the cancer. Unfortunately, these treatments can carry serious side effects. Chemotherapy can cause a variety of ailments, including hair loss, digestive problems, nausea, lack of energy and mouth ulcers.
But nanotechnologists think they have an answer for treatment as well, and it comes in the form of targeted drug therapies. If scientists can load their cancer-detecting gold nanoparticles with anticancer drugs, they could attack the cancer exactly where it lives. Such a treatment means fewer side effects and less medication used. Nanoparticles also carry the potential for targeted and time-release drugs. A potent dose of drugs could be delivered to a specific area but engineered to release over a planned period to ensure maximum effectiveness and the patient’s safety.
These treatments aim to take advantage of the power of nanotechnology and the voracious tendencies of cancer cells, which feast on everything in sight, including drug-laden nanoparticles. One experiment of this type used modified bacteria cells that were 20 percent the size of normal cells. These cells were equipped with antibodies that latched onto cancer cells before releasing the anticancer drugs they contained.
Another used nanoparticles as a companion to other treatments. These particles were sucked up by cancer cells and the cells were then heated with a magnetic field to weaken them. The weakened cancer cells were then much more susceptible to chemotherapy.
It may sound odd, but the dye in your blue jeans or your ballpoint pen has also been paired with gold nanoparticles to fight cancer. This dye, known as phthalocyanine, reacts with light. The nanoparticles take the dye directly to cancer cells while normal cells reject the dye. Once the particles are inside, scientists “activate” them with light to destroy the cancer. Similar therapies have existed to treat skin cancers with light-activated dye, but scientists are now working to use nanoparticles and dye to treat tumors deep in the body.
From manufacturing to medicine to many types of scientific research, nanoparticles are now rather common, but some scientists have voiced concerns about their negative health effects. Nanoparticles’ small size allows them to infiltrate almost anywhere. That’s great for cancer treatment but potentially harmful to healthy cells and DNA. There are also questions about how to dispose of nanoparticles used in manufacturing or other processes. Special disposal techniques are needed to prevent harmful particles from ending up in the water supply or in the general environment, where they’d be impossible to track.
Gold nanoparticles are a popular choice for medical research, diagnostic testing and cancer treatment, but there are numerous types of nanoparticles in use and in development. Bill Hammack, a professor of chemical engineering at the University of Illinois, warned that nanoparticles are “technologically sweet” [Source: Marketplace]. In other words, scientists are so wrapped up in what they can do, they’re not asking if they should do it. The Food and Drug Administration has a task force on nanotechnology, but as of yet, the government has exerted little oversight or regulation.
robotics
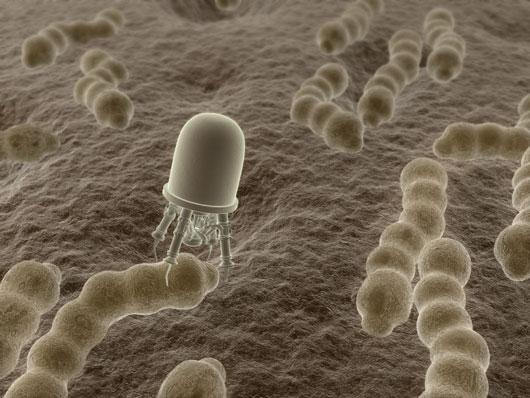
A mechanical white blood cell attacks bacteria. The bacteria cannot develop immunity to mechanical devices as it would towards a drug
Nanotechnology, perhaps, has been most popularly recognized for it’s applications in robotics. Nano-robotics, although having many applications in other areas (such as particle manipulation and, has the most useful and variety of uses in medical fields.
Drugs have been shown to be effective during treatment and so has surgery. However, both are only temporary. We do not have much control over the drugs that have entered our body. As mentioned in the “Applications in Drugs and Therapeutics” page, nanotechnology can play an important role by being used for designing drug delivery systems.
Nanorobots, once fully developed, will be more effective than drugs. This is because nanobots cab always be present in the body, fighting off pathogens such as viruses and tumors. Nanorobots will not require any additional treatment and will become relatively cheap after development.
Some of the potential applications for nano-robotics in medicine include early diagnosis and targeted drug delivery for cancer, biomedical instrumentation, surgery, pharmacokinetics, monitoring of diabetes, and health care. Medical nanotechnology in the future will use nanorobots injected into the patient to perform treatments at cellular levels
Some other possible applications using medical nanorobots are as follows:
· To cure skin diseases, a cream containing nanorobots may be used. This cream would remove the right amounts of dead skin cells, remove excess oils which may cause oily skin, insert missing oils, apply the specifically right amounts of natural moisturizing compounds. Dermatological problems would thus be avoided or removed.
· A mouthwash full of water and smart nanorobots could identify and destroy pathogenic bacteria, particles of food, plaque, or tartar, while allowing the harmless flora of the mouth to flourish. Being suspended in liquid and able to swim about, devices would be able to reach surfaces beyond reach of toothbrush bristles or the floss fibers. As short-lifetime medical nano-devices, the bots could be built to last only a few minutes in the body before falling apart into materials of the sort found in foods (such as fibers and other organic compounds). This would not cause any toxic harmful effects in the body, and there would be no need for toothbrushes.
· Medical nanodevices could augment the immune system by finding and disabling unwanted bacteria and viruses. When an invader is identified, it can be punctured, letting its contents spill out and ending its effectiveness. If the contents were known to be hazardous by themselves, then the immune machine could hold on to it long enough to dismantle it more completely. With even more innovation, pathogens could be broken down into simple substances such as oxygen and extra cellular material which can be used for benefit of the body!
· Devices working in the bloodstream could nibble away at arteriosclerotic deposits, widening the affected blood vessels. Various nano-devices could restore the strength of the arteries and veins. With such applications, many heart attacks would be prevented.
More Background on Nanotechnology:
| Nanotechnology Basics – For students and other learners | |
| Managing Magic – A brief overview of the challenges posed by advanced nanotechnology | |
| Nanotechnology on an Upward Slope – An online PowerPoint presentation | |
| Turn on the Nanotech High Beams – An essay published by Future Brief | |
| Nano Simulation – A way to visualize what is meant by molecular manufacturing | |
| Debating the Future of Nanotechnology – Perspective from the Foresight Institute | |
| Safe Utilization of Advanced Nanotechnology – One of the founding papers of CRN | |
| 5-Minute Nanosystems– A quick summary of Eric Drexler’s foundational work on nanotechnology | |
| Nanotechnology Press Kit – Compiled and published by Nanotechnology Now |

Lycopene and fish oil are two substances known for their beneficial properties. The first one, a carotene contained in red fruits such as tomatoes, has been shown to lower the risk of developing digestive track cancers. The second one possesses anti-inflammatory properties thanks to its high content in polyunsaturated fatty acids, such as eicopentaenoic and docosahexaenoic acids
read all at
http://www.chemistryviews.org/details/news/2720971/It_Takes_Two_to_a_Healthy_Colon.html
Fibristal (ulipristal acetate)
|
|
|