
Spiders are nature’s pest controllers. These eight-legged, web-forming arachnid predators are equipped with two venom glands full of valuable chemicals designed to kill insect prey. Such compounds, from small organic molecules to complex structures such as acylpolyamines, neuropeptides and enzymes, are precious ligands that target several biological receptors. Since insect receptors are not substantially different from those of humans and other mammals, the majority of the molecules contained in spider venom could also target human receptors.

|
The potential medical uses of spider venoms are largely due to their selectivity and affinity for ion channels.
|
The potential medical uses of spider venoms are largely due to their selectivity and affinity for ion channels [proteins that allow ions to cross cell membranes] and other receptors. This makes them suitable for studying cell function and for designing therapeutic drugs. As an example, the venom of the theraphosid Grammostola spatulata from South America contains a peptide, GsMtx-4, that blocks stretch-activated ion channels. These channels are sensitive to muscle contraction and blood pressure and play an important role in coordinating a heartbeat. Potentially, GsMtx-4 could be used to prevent atrial fibrillation after a heart attack and to treat cardiac patients.

LIPID BOUND Like related peptide toxins, GsMTx4 has a highly hydrophobic face (green). The cysteines that make up the protein’s core are colored yellow, and positive and negative residues are shown in blue and red, respectively.
NATURE ©2004
Peptides make up a substantial part of spider venom, and modulate ionic currents across Ca2+, Na+, or K+ ion channels. Some spider peptides can discriminate between ion channel subtypes and several will inhibit peripheral neurons, the nerve cells that are associated with supplying sensation to the skin and skeletal muscles. Spider toxins that block the neuronal Ca2+ ion channel could prove important for the treatment of chronic pain.
A special group of the spider peptides have a mixed hydrophilic-hydrophobic nature – they are amphipathic. These form ![]() -helical structures that insert into cell membranes to form pores, resulting in loss of cell function. Although most of these peptides will destroy red blood cells, they could potentially be used in topical applications, such as antibacterial coatings for medical implants, in inhibiting the growth of oral bacteria associated with tooth decay and early plaque formation and in treating skin infections.
-helical structures that insert into cell membranes to form pores, resulting in loss of cell function. Although most of these peptides will destroy red blood cells, they could potentially be used in topical applications, such as antibacterial coatings for medical implants, in inhibiting the growth of oral bacteria associated with tooth decay and early plaque formation and in treating skin infections.
Venom peptides contain a common basic structure called a ‘cysteine knot,’ a tangle of protein chains and disulfide bridges that gives them an excellent molecular stability. Also, the small organic components of spider venom, such as organic acids, amines, nucleic acids and amino acids, are thought to stabilise the mixture and enhance the delivery and effectiveness of the peptides.
Of all the venom components, the acylpolyamines represent the vast majority of the molecules in the mixture. These have been shown to suppress epileptic activity in brain tissue. They can also act as pain-killers, by blocking capsaicin receptor channels, non-selective cation channels in sensory neurons that respond to pain-causing stimuli. Moreover, brain damage caused by restricted blood flow, for example during a stroke, can be prevented with acylpolyamines. The compounds work by blocking Ca2+ voltage-gated ion channels or preventing glutamate release, both of which are implicated in neuronal death.
Finally, enzymes and large protein components of spider venoms are of special medical importance. For example, the neurotoxic protein ![]() -latrotoxin, from the black widow spider, causes massive neurotransmitter release. Similarly, an active enzyme in the venom of the brown recluse spider is sphingomyelinase D, which degrades cell membranes and causes painful lesions to develop. Another component of brown recluse spider venom, hyaluronidase, belongs to a family of compounds that have shown medical potential as tumour treatments.
-latrotoxin, from the black widow spider, causes massive neurotransmitter release. Similarly, an active enzyme in the venom of the brown recluse spider is sphingomyelinase D, which degrades cell membranes and causes painful lesions to develop. Another component of brown recluse spider venom, hyaluronidase, belongs to a family of compounds that have shown medical potential as tumour treatments.
Most spider species are harmless to humans, so peptides or drug molecules from these spiders are likely to be safe. By modifying the molecular surfaces and active sites of peptides and enzymes from spiders, whilst keeping the spider scaffold, it is possible to gain specificity and/or affinity for a given receptor. Therefore, acylpolyamines, peptides and enzymes from spider venoms represent an interesting source of molecules for the design of novel pharmaceutical drugs.
References
Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs
G Estrada, E Villegas and G Corzo, Nat. Prod. Rep., 2007,
DOI: 10.1039/b603083c
Applications of Spider Venom
Interest in potential agricultural and medical uses of spider venom is largely due to its selectivity in species and site of action. Current research centres around exploring the development of pesticides and drugs for treating cardiac patients.
Pesticides

Components in the neurotoxic venom of an Australian funnel-web spider have been found to be specific for insects such as cockroaches, crickets, fruit-flies and the Helicoverpa armigera moth which destroys cotton crops. Targeting specific species prevents the accidental killing of other insects. This selectivity also means that the pesticide is harmless to other organisms so there would be no danger if it entered the food chain. The compounds in venom are environmentally friendly and the development of resistance to a spider venom pesticide would be slow. Traditional chemical pesticides do not tend to be species specific, are toxic to humans in large amounts and insects develop resistance towards them relatively fast so it is easy to see why pesticides based on spider venom are attractive.
Prevention of Atrial Fibrillation
The venom of the Chile Rose tarantula (Grammostola spatulata) from South America contains an active protein, GsMtx-4, which blocks ion channels that are stretch activated. These channels are therefore sensitive to muscle contraction and blood pressure and play an important role in co-ordinating a heartbeat. A heart attack causes these ion channels to open and release chemicals which interfere with the heart rhythm leading to atrial fibrillation. Fibrillation is when the upper heart chambers (the atria) contract rapidly and prevent sufficient blood from entering the lower chambers (the venticles). It is fibrillation which often causes the death of a heart attack victim, not the attack itself so GsMtx-4 could be utilised in a potentially life-saving drug which prevents fibrillation. GsMtx-4 is ineffective on the normal unstretched heart so side effects should be small or even non-existent. The venom from the Chile Rose spider is also harmless to humans which constitutes an extra safety precaution.
Prevention of Brain Damage
Oxygen deprivation caused by events such as stroke or excessive smoke inhalation can result in nerve cell damage in the brain. Glutamate is a neurotransmitter in the human brain and large amounts of it are released by these damaged neurons causing the death of neighbouring nerve cells. The Holena curta funnel-web spider produces a venom containing the active ingredient HF-7 which blocks receptors on the nerve cell membranes and prevents glutamate production. A drug developed using this compound could therefore limit brain damage for stroke victims.
















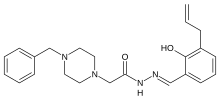

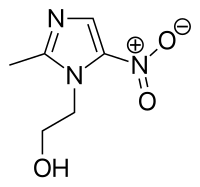



.jpg)
.jpg)


















