
B Family Album -Structural Biology: First structures of class B G protein-coupled receptors may aid drug hunts



Health Canada has approved two GlaxoSmithKline (GSK) drugs, Tafinlar(TM) (dabrafenib mesilate) and Mekinist(TM) (trametinib), for patients with unresectable or metastatic melanoma. Dabrafenib mesylate, which is a BRAF-inhibitor, is indicated as a monotherapy oral treatment for unresectable melanoma or metastatic melanoma in adult …http://www.gsk.ca/english/html/media-centre/2013-07-24.html

Iron oxide nanoparticles precisely direct stem cells to damage tissues, thereby increasing their therapeutic potential
http://www.chemistryviews.org/details/news/5039301/Iron_Oxide_Nanoparticles_Show_the_Way.html
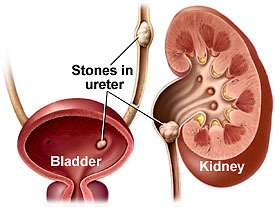
The discovery of a gene’s function in E. coli and other bacteria might lead to a probiotic to prevent calcium-oxalate urinary stones, the most common type of kidney stone. Human cells can’t metabolize oxalate and high concentrations can result in painful blockages of the urinary tract.
selumetinib
Array Biopharma To Report Top-line Results From ARRY-502 Asthma Trial
Sacramento Bee
AstraZeneca began a pivotal trial with selumetinib (an Array-invented drug) in patients with thyroid cancer in May 2013 and expects to begin a Phase 3 trial in patients with non-small cell lung cancer during the second half of 2013. Three other Array …
http://www.sacbee.com/2013/07/22/5586413/array-biopharma-to-report-top.html
Selumetinib (AZD6244) is a drug being investigated for the treatment of various types of cancer, for example non-small cell lung cancer (NSCLC).

The gene BRAF is part of the MAPK/ERK pathway, a chain of proteins in cells that communicates input from growth factors. Activating mutations in the BRAF gene, primarily V600E (meaning that the amino acid valine in position 600 is replaced by glutamic acid), are associated with lower survival rates in patients with papillary thyroid cancer. Another type of mutation that leads to undue activation of this pathway occurs in the gene KRAS and is found in NSCLC. A possibility of reducing the activity of the MAPK/ERK pathway is to block the enzyme MAPK kinase (MEK), immediately downstream of BRAF, with the drug selumetinib. More specifically, selumetinib blocks the subtypes MEK1 and MEK2 of this enzyme.[1]
KRAS mutations appear in 20 to 30% of NSCLC cases and about 40% of colorectal cancer.[1]
A Phase II clinical trial about selumetinib in NSCLC has been completed in September 2011;[3] one about cancers with BRAF mutations is ongoing as of June 2012[update].[4]
|displayauthors= suggested (help) edit|displayauthors= suggested (help) editmore info…………………………………….
AZD-6244 (Selumetinib) is an orally-available, aminobenzimidazole-based, allosteric inhibitor of MEK1 kinase with an IC50 of 14 nM. [1] IC50 concentrations of
In cellular growth assays, AZD-6244 was more potent in cell lines containing activating B-Raf and Ras mutations, with IC50 values ranging from 59 to 473 nM. In HT-29 and Malme-3M cell studies, AZD-6244 was found to induce G1-S cell cycle arrest, inducing apoptosis after a 2-day incubation period. [1] In Colo-205 xenografts, AZD6244 induced increased levels of cleaved caspase-3, indicating apoptosis. [2]
In diffuse large B-cell lymphoma (DLBCL) lines, nanomolar concentration of AZD-6244 effectively downregulated MEK/ERK target substrates, including c-Myc, Mcl-1, and Bcl-2. [3]
Technical information:
| Chemical Formula: | C17H15BrClFN4O3 | |
| CAS #: | 606143-52-6 | |
| Molecular Weight: | 457.68 | |
| Appearance: | White | |
| Chemical Name: | 6-(4-bromo-2-chlorophenylamino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-3H-benzo[d]imidazole-5-carboxamide | |
| Solubility: | Up to 100 mM in DMSO | |
| Synonyms: | AZD-6244, AZD 6244, AZD6244, Selumetinib, Selumetinib sulfate, NSC-748727, ARRY-142886 |
Reference:
| 1. | Yeh et al., Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin. Cancer Res. 2007, 13, 1576-1583 Pubmed ID: 17332304 |
| 2. | Davies et al., AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol. Cancer Ther. 2007, 6, 2209-2219. Pubmed ID: 17699718 |
| 3. | Bhalla et al., The novel anti-MEK small molecule AZD6244 induces BIM-dependent and AKT-independent apoptosis in diffuse large B-cell lymphoma. Blood, 2011, 118(4), 1052-1061. Pubmed ID: 21628402 |



A compound is being investigated as a Type II diabetes treatment by Lexicon Pharmaceuticals, although it is in an early stage of development
 LX4211
LX4211
A compound is being investigated as a Type II diabetes treatment by Lexicon Pharmaceuticals, although it is in an early stage of development.
LX4211 is not selective for just sodium glucose co-transporter-2, or SGLT-2 – it also inhibits SGLT-1.1 Inhibiting this second transporter, responsible for the absorption of glucose in the intestines, it also results in an increase in the release of glucagon-like peptide-1 (GLP-1), but might be combated by administering the dual inhibitor in combination with a dipeptidyl peptidase-4 (DPP-4) inhibitor to prevent it being activated.
– See more at:
http://www.manufacturingchemist.com/technical/article_page/Diabetes__LX4211/88179

Dr Reddy’s Laboratories Ltd new patent on
Preparation of lubiprostone
Jackson, Mark; Dahanukar, Vilas Hareshwar; Joseph, Suju Chuttippari; Eda, Vishnu Vardhana Verma Reddy; Ramdas, Sandip Khobare
US 20130184476, 18-JUL-2013
| IN2011CH2389 | 13-JUL-2011 priority |
NCT01674530, Phase 3
_____________________________________________________
general info in public domain
Lubiprostone (rINN, marketed under the trade name Amitiza) is a medicationused in the management of chronic idiopathic constipation and irritable bowel syndrome. It was approved by the U.S. Food and Drug Administration (FDA) for this purpose on 31 January 2006.
Lubiprostone is used for the treatment of chronic constipation of unknown cause in adults, as well as irritable bowel syndrome associated with constipation in women.
As of 20 July 2006, Lubiprostone has not been studied in children. There is current research underway to determine the efficacy in postoperative bowel dysfunction, and opioid-induced bowel dysfunction.
Synthesis:Sobrera, L. A.; Castaner, J. (2004). Drugs of the Future 29 (4): 336.

PA 824
The search for a new tuberculosis drug after many decades and first time through a unique model of open drug discovery programme may finally bear fruits in near future, with India all set for the launch of the phase II clinical trial of the drug candidate.
The drug, coming through the Open Source Drug Discovery (OSDD) programme by Council of Scientific and Industrial Research (CSIR), will go for the clinical trials on drug-resistant TB patients in India very soon. The process of filing for permission from the Drug Controller General of India (DCGI) is on and public sector LRS Hospital for Respiratory and Infectious Diseases, New Delhi, has been selected for trials. This phase II trials will involve around 250-300 patients, sources said.

The drug candidate, Pa824, was synthesised in India long ago. After a series of ownership changes, the molecule was licensed to CSIR for further development now.
http://www.pharmabiz.com/NewsDetails.aspx?aid=76568&sid=1
Tuberculosis (TB) is one of the leading infectious diseases in the world, with approximately one-third of the world’s population harboring the causative agent, Mycobacterium tuberculosis (Mtb). Though previously a disease associated with aristocratic societies, TB is now predominantly a third-world disease, particularly affecting Asian communities and sub-Saharan Africa. Mtb isolates are increasingly resistant to drug therapies: multidrug-resistant TB (MDR TB) or more severely, extensively drug-resistant TB (XDR TB). As a consequence of these emerging strains, it is becoming increasingly apparent that novel drugs are necessary to combat Mtb infections.

PA 824 is an experimental anti-tuberculosis drug.[1][2] The bicyclic nitroimidazole like molecule PA-824 has got a very complex mechanism of action active against both replicating and hypoxic, non-replicating Mycobacterium tuberculosis.Microarray analysis of the mode of action of PA-824 showed a puzzling mixed effect both on genes responsive to both cell wall inhibition (like isoniazid) and respiratory poisoning (like cyanide). The aerobic killing mechanism of this drug appears to involve inhibition of cell wall mycolic acid biosynthesis through an as yet unknown molecular mechanism.The respiratory poisoning through nitric oxide release seemed to be a crucial element of anaerobic activity by PA-824. The effect of PA-824 on the respiratory complex under hypoxic non-replicating conditions was also manifested in a rapid drop in intracellular ATP levels, again similar to that observed by cyanide treatment.[3]PA-824 recently was shown to be safe, well tolerated, and efficacious at doses of 100–200 mg daily in a dose-ranging study among drug-sensitive, sputum smear–positive, adult pulmonary TB patients [4]


QSAR modeling of the nitroimidazole PA-824. Shown are two hydrogen bond acceptors (green), one hydrogen bond donor (purple), and one hydrophobe (aqua). Credit: NIAID
picked up from a japanes site
Available at:
It is said that drug discovery of target-based drug discovery is a paradigm of the past 20 years, was not very effective in what percent of one hundred billion yen of investment. Why did not work so much? You know why a high failure probability of the conventional method, we propose the transformation of drug discovery techniques to improve productivity in this case.
For example, at the stage of target selection, many researchers trying to aim at compounds selective, but as can be seen from kinase cancer research, and shows a medicinal action at the same time a plurality of signals by the target multi- If you want to demonstrate to that you often. Further, it tends to require a clear MOA leading to efficacy in pharmaceutical companies, but the MOA is used to not be fully described as drug mental illness often actually in practice. Movement of proteins in vivo actually that it is not so simple many MOA to be described coherently At first glance also, also, may be used instead of different views depending on the specifications of interpretation in practice.
If you prove that it is selective for off-target of 100 kinds about the compound that is optimized in a single mechanism, if there is action on targets unknown other, it is that there is a clinically significant after we In some cases, it can be seen. Next is to evaluate and to build the flow of screening MOA and target Once you have decided, but it is also not necessarily the one that bridges the screening and clinical actually.
Case of cancer cells, and so extrapolate clinical efficacy in inhibition of proliferation activity, but are those far removed from the actual in vivo in most cases, the process itself is continuously evaluated by routine work ends as a waste of time not money. If you choose to use compound somehow, animal disease model is not possible to accurately predict clinical outcome. Uncertainties like this a lot, theme 97% never fail downright non-clinical. Rather than biology and disease clearly, feeling had focused on the process is undeniable drug discovery paradigm of current. Important thing originally, is to to centered on principles and appropriate science focused on disease in clinical, it is to re-build process on it.
Reorganization research and development are important, it is not able to change the nature of the disease where you have reorganized matter. Therefore, it is a secondary or if the organization. The new research paradigms consider the process to focus on the disease, it is necessary to apply the three principles. One of them is the process of science-based.Science is made up by systematic law, which is evidenced by the accumulation of observation and experiment without prejudice. It’s that you have to often is that is do we need to note here, discussions would begin while not to suspect the hypothesis and doctrine that are major premise.
For example, the earth if the major premise that it is a flat, what’s there in the flat world of another fall from the tip of the flat earth?Question that will come out. However, if you can dispel the assumption by the belief that there is no basis to recognize the shape of the earth, it is possible to pursue science in the right direction in a totally different perspective. This same is true for drug discovery research. For example, if a certain phenomenon is to work, it would no longer consider the direction of the other.
Cause of schizophrenia may be mentioned a theory that based on the excess dopamine hyperactivity as an example.May develop the symptoms of schizophrenia-like D amphetamine is allowed to release dopamine, this concept is the basis that it has a D2 antagonism dopamine psychotropic drugs many. However, it is said, not the D amphetamine contraindicated, psychotropic drugs long-term treatment is also lead to enhanced dopamine system schizophrenia many patients. However, the fact that you have these, never to be little consideration on the grounds that would be go against the doctrine of dopamine hypothesis. In other words, the doctrine would be to blind us to the alternative hypothesis essentially.
This same is true with respect to Alzheimer’s.When the doctrine of a certain hypothesis is treated as the principles, facts that go against the doctrine is ignored, hypothesis another is neglected, progress of science’s delayed in order to look away from the truth. Hypothesis became a doctrine becomes absolute only and is deified as the Bible. Fact defeat the hypothesis that even came out after another, the cause interpreted as another factor, never wake up until push forward until you collapse in clinical late as a result. Hypothesis that build on doctrine the wrong does not lead only in the wrong direction all.
Simply because I admit to know that the earth is a sphere, a new way of science he is cut open. In addition, reductionism approach dominant also should be noted in the discussion of science. Element has a plurality of by influencing the complex in vivo, originally, it is not possible to understand the living body to focus on a single element. For example, to understand the engine of the car, there is a need to disconnect the engine from the mirror and the door, however, they are not able to understand the engine itself and accidentally disconnect without knowing the function of important and bolts cylinder engine. Multi-target has been spotlighted in the drug synergy effect by the action of more than one is because the expected effect even insufficient for single target. That it kept asking the research many times, to re-examine the design of the experiment is important in decision-making, but is in surprisingly poor. Schizophrenia negative symptoms model of phencyclidine-induced and model of cerebral infarction has become a problem much that there is no reproducibility by the lab.
In the process of science-based, it will continue to refine while obtaining conclusive evidence to do the practice and discussion is important. Clinical success rate is low, it sifted the hypothesis as much as possible in the process of advancing the research, better should remain. The second principle is that the customers know. Be patient with the customer for the pharmaceutical companies. It is not possible to produce products that are competitive by knowing the limit disease, condition, existing drugs. Even on the researchers know the needs of the patient, taking into closer contact with the doctor is important. Principles of the third, is to understand the risks. The acceptable risk, it is not what we deal with the risk, that is, whether to undertake the corresponding risk, the only pass to avoid the discussion if not. First of all drug discovery are those difficult, many programs to fail in the mid-way is typical for the pharmaceutical companies.
However, it should be avoided risk, such as notice and a predictable if you even care, because he turn away the failure of such a future, because it should not be happening that failure waiting to happen after we some. A few years ago, pharmaceutical companies some had considered mGluR5 antagonists as an anti-anxiety drug. This MOA was expected as much anti-anxiety drug, but the side effects of psychotic disorders such as hallucinations and delusions has been concern on the other hand. However, was never to be fully verified until the compound drop in its side effects in Phase 1 this concern. Many pharmaceutical companies are in was not able to deal with known risks.
Any pharmaceutical company, and the last would complete the study because of the common “because other companies are doing” and, nobody did not want to press the stop button. Pharmaceutical companies had been wiped out by the results of the problem banzai charge of pharmaceutical companies which can be called reckless risk-taking took place waiting to happen in clinical trials. To avoid this situation, the practice of the process of due diligence scientific is the essential. Inspection with no bias due to opposition support and opinion is carried out in this process. And verified by experiment hypothesis that there is sufficient scientific reason to support (1) project, if you can dispel the uncertain surface with a (2) simple additional experiments, and (3) key in this process it is determined Uruka about, you can reduce the risk of later on in (4) checkpoint. Researchers lament “factor of the disease is too complex” and the “because there is no way of doing the other”, the researchers would start the research been sticking to the one target.
However, you should know that a little time, to discuss the clinical symptoms of the disease, you only need to discuss the screening method of Japan before, and there is also a problem that can be resolved