Uprosertib (GSK-2141795)
GSK 2141795C
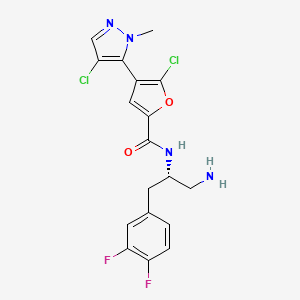
N-[(1S)-1-(aminomethyl)-2-(3,4-difluorophenyl)ethyl]-5-chloro-4-(4-chloro-1-methyl-1H-pyrazol-5-yl)furan-2-carboxamide
N-[(2S)-1-amino-3-(3,4-difluorophenyl)propan-2-yl]-5-chloro-4-(4-chloro-2-methylpyrazol-3-yl)furan-2-carboxamide
2-Furancarboxamide, N-[(1S)-2-amino-1-[(3,4-difluorophenyl)methyl]ethyl]-5-chloro-4-(4-chloro-1-methyl-1H-pyrazol-5-yl)-
Λ/-{(1 S)-2-amino-1-r(3,4-difluorophenyl)methyllethyl}-5-chloro-4-(4- chloro-1-methyl-1H-pyrazol-5-yl)-2-furancarboxamide
Cas 1047634-65-0 (GSK-2141795); BASE
CAS 1047635-80-2 (GSK-2141795 HCl salt)
Synonym: GSK-2141795; GSK2141795; GSK 2141795; GSK795; GSK-795; GSK 795. Uprosertib. UNII ZXM835LQ5E
IUPAC/Chemical name:
N-((S)-1-amino-3-(3,4-difluorophenyl)propan-2-yl)-5-chloro-4-(4-chloro-1-methyl-1H-pyrazol-5-yl)furan-2-carboxamide
C18H16Cl2F2N4O2
Exact Mass: 428.06184
Molecular Weight: 429.25
Elemental Analysis: C, 50.37; H, 3.76; Cl, 16.52; F, 8.85; N, 13.05; O, 7.45
Mechanims of Action:Akt inhibitor
Indication:Cancer Treatment
Drug Company:GlaxoSmithKline
PHASE 2… CANCER
Uprosertib, also known as GSK2141795 and GSK795, is an orally bioavailable inhibitor of the serine/threonine protein kinase Akt (protein kinase B) with potential antineoplastic activity.
The National Cancer Institute (NCI) is evaluating the compound in phase II clinical studies for the treatment of endometrial carcinoma and multiple myeloma in combination with trametinib.
GSK-2141795, an oral AKT inhibitor, is in early clinical trials at GlaxoSmithKline for the treatment of solid tumors and lymphoma. The company is conducting phase II clinical trials for the treatment of patients with BRAF wild-type mutation melanoma and for the treatment of recurrent or persistent cervical cancer in combination with trametinib.
Akt inhibitor GSK2141795 binds to and inhibits the activity of Akt, which may result in inhibition of the PI3K/Akt signaling pathway and tumor cell proliferation and the induction of tumor cell apoptosis. Activation of the PI3K/Akt signaling pathway is frequently associated with tumorigenesis and dysregulated PI3K/Akt signaling may contribute to tumor resistance to a variety of antineoplastic agents.
| QC data: |
PATENT
| PATENT | SUBMITTED | GRANTED |
|---|---|---|
| Inhibitors of AKT Activity [US2011071182] | 2011-03-24 | |
| INHIBITORS OF Akt ACTIVITY [US2010267759] | 2010-10-21 | |
| INHIBITORS OF AKT ACTIVITY [US2009209607] | 2009-08-20 | |
| INHIBITORS OF Akt ACTIVITY [US2010041726] | 2010-02-18 |
More information about this drug
The chemical structures of Afuresertib (GSK-2110183) and GSK-2141795 are very similar as shown below:
 |
Fig 1. chemical structures of Afuresertib (GSK-2110183) and GSK-2141795
PATENT
WO 2008098104 OR EP2117523
http://www.google.com/patents/EP2117523A1?cl=en
Scheme 2
11-1 I-2
II-3 II-4
Reagents: (a) PyBrop, (i-Pr)2NEt, 1 ,1-dimethylethyl (2-amino-3- phenylpropyl)carbamate, DCM, RT; (b) 5-(5,5-dimethyl-1 ,3,2-dioxaborinan-2-yl)-1- methyl-1 H-pyrazole, K2CO3, Pd(PPh3)4, dioxane/H2O; (c) TFA / DCM, RT.
Preparation 7
Preparation of 5-(5,5-dimethyl-1 ,3,2-dioxaborinan-2-yl)-1 -methyl-1 H-pyrazole
To a solution of 1 -methyl pyrazole (4.1 g, 50 mmole) in THF (100 ml.) at 00C was added n-BuLi (2.2M in THF, 55 mmole). The reaction solution was stirred for 1 hour at RT and then cooled to -78°C [J. Heterocyclic Chem. 41 , 931 (2004)]. To the reaction solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (12.3 ml_, 60 mmole). After 15 min at -78°C, the reaction was allowed to warm to 00C over 1 hour. The reaction was diluted with saturated NH4CI solution and extracted with DCM. The organic fractions were washed with H2O (2 x 100 ml_), dried over Na2SO4 and concentrated under vacuum to afford a tan solid (8.0 g, 77%) which was used without further purification. LCMS (ES) m/z 127 (M+H)+ for [RB(OH)2]; 1H NMR (CDCI3, 400 MHz) δ 7.57 (s, 1 H), 6.75 (s, 1 H), 4.16 (s, 3H), and 1.41 (s, 12H).
Example . .24
Preparation Λ/-{(1 S)-2-amino-1-r(3,4-difluorophenyl)methyllethyl}-5-chloro-4-(4- chloro-1-methyl-1H-pyrazol-5-yl)-2-furancarboxamide
a) methyl 4-(1-methyl-1H-pyrazol-5-yl)-2-furancarboxylate
A solution of methyl 4-bromo-2-furancarboxylate (470 mg, 2.29 mmol), potassium carbonate (1584 mg, 11.46 mmol), 1-methyl-5-(4,4,5,5-tetramethyl-1 ,3,2- dioxaborolan-2-yl)-1 H-pyrazole (525 mg, 2.52 mmol)[prepared according to Preparation 7] and bis-(tri-t-butylphosphine)Palladium (0) (58.6 mg, 0.12 mmol) in 1 ,4-dioxane (9.55 ml) and water (1.9 ml) was stirred at 80 0C. After 1 hr, the solution was partitioned between H2O-DCM and the aqueous phase was washed several times with DCM. The combined organic fractions were dried over I^^SOφ concentrated and purified via column chromatography (30% EtOAc in hexanes) affording the title compound (124 mg, 0.60 mmol, 26 % yield) as a white powder: LCMS (ES) m/e 206 (M+H)+.
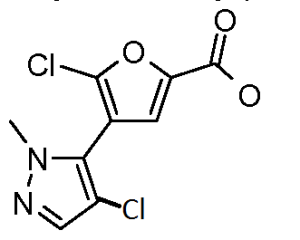
b) methyl 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylate
A solution of methyl 4-(1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylate (412 mg, 2.0 mmol) and N-chlorosuccinimide (267 mg, 2.0 mmol) in DMF (10 ml.) was heated at 75 0C for 30 minutes. Another batch of N-chlorosuccinimide (267 mg, 2.0 mmol) was added. After 1 hr, the mixture was concentrated and purified using silica gel and eluting with 0-55% ethyl acetate / hexane to afford the title compound as a white solid (225 mg, 0.82 mmol, 71 % yield) : LCMS (ES) m/e 276 (M+H)+.
c) 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylic acid
A solution of methyl 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-2- furancarboxylate (224 mg, 0.82 mmol) in 6N sodium hydroxide (1.36 ml, 8.2 mmol) and tetrahydrofuran (5 ml) was stirred at 70 0C in a sealed tube for 1 h. The resulting solution was cooled and then partitioned between H2O-DCM. The aqueous phase was adjusted to pH ~4 and then washed several times with DCM. The combined organic fractions were dried over Na2SO4 and concentrated affording the title compound (201 mg, 0.77 mmol, 94 % yield) as a yellow oil: LCMS (ES) m/e 262 (M+H)+.
d) 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-N-{(1S)-2-(3,4-difluorophenyl)-1- [(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}-2-furancarboxamide
To a solution of 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-2- furancarboxylic acid (200 mg, 0.77 mmol)[prepared according to the procedure of Preparation 6], 2-[(2S)-2-amino-3-(2,4-difluorophenyl)propyl]-1 H-isoindole-1 ,3(2H)- dione (254 mg, 0.80 mmol) and N,N-diisopropylethylamine (0.40 ml, 2.30 mmol) in DCM (10 ml) was added bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (536 mg, 1.15 mmol). After stirring at ambient temperature for 20 hrs, the mixture was concentrated and purified with silica gel column eluting with gradient (0-50% ethyl acetate/hexanes) to afford the title compounds as an off-white foamy solid (304 mg, 0.54 mmol, 71 % yield): LCMS (ES) m/e 560(M+H)+.
e) Λ/-{(1 S)-2-amino-1-[(3,4-difluorophenyl)methyl]ethyl}-5-chloro-4-(4-chloro-1- methyl-1 /-/-pyrazol-5-yl)-2-furancarboxamide
To a solution of 5-chloro-4-(4-chloro-1-methyl-1 H-pyrazol-5-yl)-N-{(1S)-2- (3,4-difluorophenyl)-1 -[(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)methyl]ethyl}-2- furancarboxamide (304 mg, 0.54 mmol) in methanol (5 ml) at 25 0C was added hydrazine (0.08 ml, 2.7 mmol) dropwise. After 12h, the solution was concentrated, dry loaded onto silica and purified by column chromatography (5% MeOH in DCM (1 % NH4OH)). The free base was converted to the HCI salt by addition of excess 4M HCI in dioxane (1 ml) to the residue in MeOH (2 ml) affording the HCI salt of the title compound as a yellow solid:
LC-MS (ES) m/z 430(M+H)+,
1H NMR (400 MHz, MeOD) δ ppm 2.91 – 3.05 (m, 2 H) 3.17 – 3.28 (m, 2 H) 3.81 (s, 3 H) 4.57 (d, J=9.60 Hz, 1 H) 7.12 (br. s., 1 H) 7.18-7.28 (m., 2 H) 7.36-7.39 (m, 1 H) 7.58 (s, 1 H).
SYNTHESIS ELABORATED
STEP A
STEP C
LCMS (ES) m/e 560(M+H)+
NOTE STRUCTURE OF 2-[(2S)-2-amino-3-(2,4-difluoro phenyl)propyl]-1H-isoindole-1,3(2H)-dione

SEE http://www.google.com/patents/WO2010093885A1?cl=en
Preparation 1
Preparation of 2-[(2S)-2-amino-3-(3,4-difluorophenyl)propyl1-1 /-/-isoindole-1 ,3(2H)-dione a) 1 ,1-dimethylethyl [(1 S)-2-(3,4-difluorophenyl)-1-(hydroxymethyl)ethyl]carbamate
To a solution of Λ/-{[(1 ,1-dimethylethyl)oxy]carbonyl}-3,4-difluoro-L-phenylalanine (2.0 g, 6.7 mmol) in THF (35 ml.) at 0 0C stirred was added BH3-THF (30 ml_, 30 mmol- 1 M in THF). After 12h, the reaction was quenched with AcOH:MeOH (1 :4, 20 ml.) and partitioned between saturated aqueous NaHCO3 and CHCI3. The aqueous phase was then extracted several times with CHCI3. The combined organic fractions were concentrated and the resulting white solid (7.0 g, 74%) used without further purification: LCMS (ES) m/e 288 (M+H)+.
b) 1 ,1-dimethylethyl {(1 S)-2-(3,4-difluorophenyl)-1-[(1 ,3-dioxo-1 ,3-dihydro-2/-/-isoindol-2- yl)methyl]ethyl}carbamate
To a solution of 1 ,1-dimethylethyl [(1 S)-2-(3,4-difluorophenyl)-1-
(hydroxymethyl)ethyl]carbamate (2.65 g, 9.22 mmol), polymer bound triphenylphosphine (5.33 g, 1 1.5 mmol, 2.15 mmol/g) and phthalimide (1.63 g, 10.9 mmol) in THF (50 ml.) at 25 0C was added diisopropyl azodicarboxylate (1.85 ml_, 11.3 mmol). After stirring at RT for 1 h, the reaction solution was filtered and concentrated. The residue was adsorbed onto silica and purified via column chromatography to yield product (0.33 g) as a white solid: LCMS (ES) m/z 417 (M+H)+.
c) 2-[(2S)-2-amino-3-(3,4-difluorophenyl)propyl]-1 H-isoindole-1 ,3(2H)-dione
To a solution of 1 ,1-dimethylethyl {(1S)-2-(3,4-difluorophenyl)-1-[(1 ,3-dioxo-1 ,3- dihydro-2H-isoindol-2-yl)methyl]ethyl}carbamate (0.33 g, 0.79 mmol) in CHCI3:MeOH (10:3, 13 mL) at RT was added 4M HCI in dioxane (5 mL, 20 mmol). After 12h, the solvents were removed and affording the title compound (0.29 g, quant.) as a white HCI salt which was used without further purification: LCMS (ES) m/z 317 (M+H)+.
FINAL STEP
yl)methyl]ethyl}-2-furancarboxamide in methanol (5 ml) AND hydrazine …..N-{(1S)-2-amino-1-[(3,4-difluorophenyl)methyl]ethyl}-5-chloro-4-(4-chloro-1-methyl-1Hpyrazol-5-yl)-2-furancarboxamide.
Example 127
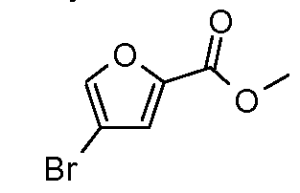
a) methyl 4,5-dibromo-2-furancarboxylate
To a solution of 4,5-dibromo-2-furancarboxylic acid (25 g, 93 mmol) in methanol (185 ml) was added sulfuric acid (24.7 ml, 463 mmol). The resulting solution stirred at 50 0C over 12h. The solution was partitioned between H2O-DCM and the aqueous phase was washed several times with DCM. The combined organic fractions were dried over I^^SOφ concentrated and used directly without further purification providing methyl 4,5-dibromo-2-furancarboxylate (23.67 g, 83 mmol, 90 % yield), LCMS (ES) m/e 283, 285, 287 (M, M+2, M+4)+.b) methyl 4-bromo-2-furancarboxylate Br
To a solution of methyl 4,5-dibromo-2-furancarboxylate (3.3 g, 1 1.62 mmol) in tetrahydrofuran (46 ml) at -40 0C was added isopropylmagnesium chloride (6.97 ml, 13.95 mmol). After 1 h, Water (11 ml) was added and the solution warmed to 25 0C. The reaction mixture was then partitioned between H2O-DCM and the aqueous phase was washed several times with DCM. The combined organic fractions were dried over Na2SOφ concentrated and purified by column chromatography (3% EtOAc in hexanes) affording methyl 4-bromo-2-furancarboxylate (1.4 g, 6.49 mmol, 56 % yield) as a yellow solid: LCMS (ES) m/e 205, 207 (M, M+2)+.
c) methyl 4-bromo-5-chloro-2-furancarboxylate
A solution of methyl 4-bromo-2-furancarboxylate (1.4 g, 6.83 mmol) and NCS (0.912 g, 6.83 mmol) in N,N-dimethylformamide (13.7 ml) was stirred in a sealed tube for 1 h at 100 0C. After 1 h, the solution was partitioned between DCM- H2O and the aqueous phase was washed several times with DCM. The combined organic fractions were dried over I^^SOφ concentrated and purified via column chromatography (2-10% EtOAc in hexanes) affording methyl 4-bromo-5-chloro-2- furancarboxylate (1.348 g, 5.12 mmol, 75 % yield) as a white solid: LCMS (ES) m/e 238, 240, 242 (M, M+2, M+4)+.
d) methyl 5-chloro-4-(1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylate
A solution of methyl 4-bromo-5-chloro-2-furancarboxylate (1.1 g, 4.59 mmol), 1-methyl-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-1 H-pyrazole (1.05 g, 5.05 mmol)[prepared according to Preparation 7], potassium carbonate (3.17 g, 22.97 mmol) and bis(tri-t-butylphosphine)palladium(0) (0.117 g, 0.23 mmol) in 1 ,4- dioxane (19.14 ml) and water (3.83 ml) was stirred at 80 0C in a sealed tube for 1 h. The reaction mixture was partitioned between H2O-DCM and the aqueous phase was washed several times with DCM. The combined organic fractions were dried over Na2SOφ concentrated and purified via column chromatography (silica, 4-25% EtOAc in hexanes) yielding methyl 5-chloro-4-(1-methyl-1 H-pyrazol-5-yl)-2- furancarboxylate (800 mg, 2.53 mmol, 55 % yield) as a yellow oil: LCMS m/e ES 240, 242 (M, M+2)+.
e) 5-chloro-4-(1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylic acid
A solution of methyl 5-chloro-4-(1-methyl-1 H-pyrazol-5-yl)-2- furancarboxylate (300 mg, 1.25 mmol) in 6N sodium hydroxide (4.16 ml, 24.93 mmol) and tetrahydrofuran (5.4 ml) was stirred at 70 0C in a sealed tube for 1 h. The resulting solution was cooled and then partitioned between H2O-DCM. The aqueous phase was adjusted to pH ~4 and then washed several times with DCM. The combined organic fractions were dried over Na2SO4 and concentrated affording 5-chloro-4-(1-methyl-1 H-pyrazol-5-yl)-2-furancarboxylic acid (267 mg, 0.59 mmol, 47 % yield) as a white foam: LCMS (ES) m/e 265 (M+H)+.
| References |
1: Dumble M, Crouthamel MC, Zhang SY, Schaber M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA, Seefeld MA, Rouse MB, Rabindran SK, Heerding DA, Kumar R. Discovery of Novel AKT Inhibitors with Enhanced Anti-Tumor Effects in Combination with the MEK Inhibitor. PLoS One. 2014 Jun 30;9(6):e100880. doi: 10.1371/journal.pone.0100880. eCollection 2014. PubMed PMID: 24978597; PubMed Central PMCID: PMC4076210.
2: Pachl F, Plattner P, Ruprecht B, Médard G, Sewald N, Kuster B. Characterization of a chemical affinity probe targeting Akt kinases. J Proteome Res. 2013 Aug 2;12(8):3792-800. doi: 10.1021/pr400455j. Epub 2013 Jul 3. PubMed PMID: 23795919.
3: Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010 Nov;19(11):1355-66. doi: 10.1517/13543784.2010.520701. Epub 2010 Sep 16. Review. PubMed PMID: 20846000; PubMed Central PMCID: PMC3244346.