http://www.fda.moph.go.th/eng/index.stm
[PDF]Regulatory Requirement for the Approval of generic Drug in Thailand …
www.jpsbr.org/index_htm_files/JPSBR14RV4029.pdf
Apr 13, 2014 – Thailand has its own drug registration format and also follows. ASEAN CTD. … Transparency in the regulatory authorities of member countries.
THAILAND PHARMACEUTICAL REGISTRATION AND APPROVAL
The Thai FDA (TFDA), one of several agencies under the Ministry of Public Health (MPH), is the regulatory body administering drugs in Thailand. The Drug Control Division of the TFDA is responsible for registration, licensing, surveillance, inspection and adverse event monitoring for all pharmaceuticals and pharmaceutical companies in Thailand. Foreign pharma companies dominate the Thai drug market. Due in part to trade negotiations, regional harmonization and positive economic trends, the pharmaceutical market in Thailand is predicted to double by 2022.There are several versions of the Drug Act currently in effect, and the Thai government is working on a revised version with updated regulations. Under the current laws, pharmaceuticals are categorized as either traditional or modern medicines, with different applications and oversight. Modern medicines are subdivided into three categories, each of which has separate registration requirements. Licenses currently do not require renewal.

link……….http://drug.fda.moph.go.th/eng/
FIRST ASEAN COUNTRY WITH A NATIONAL eCTD PROGRAM

Thai FDA intends to accept dossier in eCTD format: The Drug Regulatory Authority of Thailand (Thai FDA) has initiated the acceptance of Pilot eCTD from October 2014.Read More
eCTD requirements
http://drug.fda.moph.go.th/eng/files/2_eSubmission%20FAQ1_0921.pdf
http://drug.fda.moph.go.th/eng/files/1_TH%20Module%201%20and%20Regional%20Specification_0921_Tch.pdf
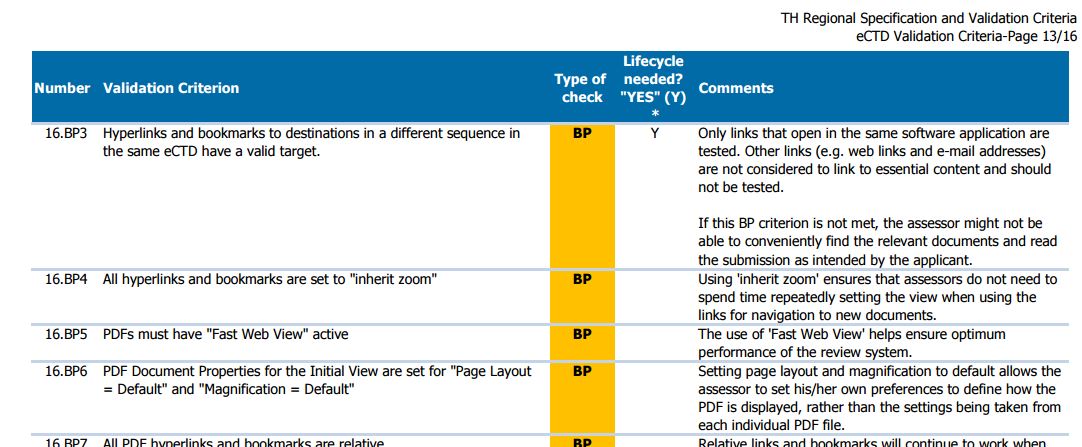
http://drug.fda.moph.go.th/eng/files/TH%20Regional%20Specification%20and%20Validation%20Criteria.pdf
Step to be followed to submit eCTD application
Taken from
 Amar Tandon
Amar TandonRegulatory Scientist at Kinapse
A) Prepare Application to get a eSubmission Identifier for every application issued. A request to the THAI FDA online service should be submitted to obtain an eSubmission identifier which will require following details.
- Licensee Number
- Description of Application
- Dosage Form
- INN or Generic Name
- Strength
- WHO ATC Code
- Sequence Type
- Application form
- CPP (In case of Importer)
The eSubmission Identifier will be issued within 10 days of application. The Applicant must then make an appointment for submission within 30 days.
B) Prepare valid application along with validation reports as per country (Thailand) specific requirement with regional eSubmission Identifier provided.
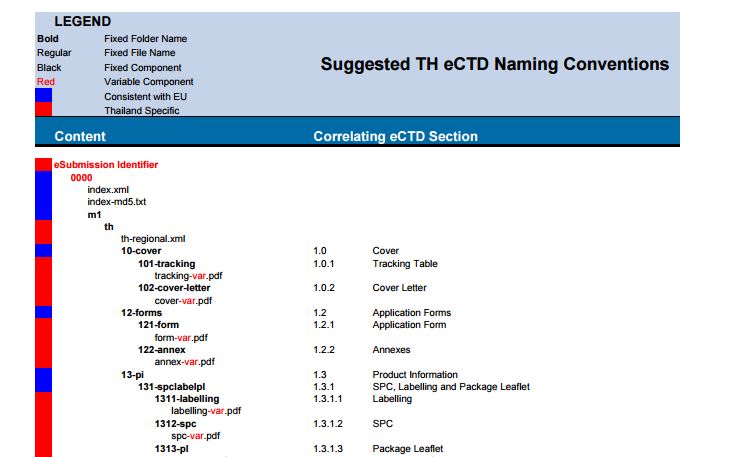
The M1 requirements to be kept in consideration while compiling the Submission.
- Enhanced granularity for each sections
- Country code is not required in filenames
- Information relating to orphan market is not mandatory
- For LCM (Life cycle management) submissions the Operation attribute should be “Replace” in Tracking Table
- Validation report should be submitted along with the sequence
- 1.3.1 Product Information has been broken down into three specific sections for Labelling, SPC and the Package leaflet. No other product types are expected. If one file is submitted for this section, it should be submitted under 1.3.1.1 Labelling.
- 1.3.1.3 Package Leaflet has been broken down into language sections for English, Thai and Other languages.
- It is recommended that separate files should be submitted for each language.
- Applicants can re-use the content submitted in other regions (including STF).
- The identifier is a combination of a letter and seven digits.
- Working documents are not needed and do not need to be provided within the eCTD framework for Thailand
- Section 1.5.2 “Information for Generic, ‘Hybrid’ or Bio-similar Applications” has been broken down into three sections and given a section number to make expectations and cross referencing clearer.
- Only one file should be provided for 1.6 Environmental Risk Assessment. It is not allowed to provide content in both 1.6.1 and 1.6.2.
- During lifecycle, 1.8.2 Risk management plan should always use the lifecycle operator replace.
C) Dispatch Activity Delivery of the application at Thai FDA in CD/DVD (make an prior appointment with HA at drug_esubmissions@fda.moph.go.th
Thai FDA has proposed a set of media formats to be used while submission of eCTD
- (CD-R) i.e. Compact Disc-Recordable
- Digital Versatile Disc-Random Access Memory (DVD-RAM)
- Digital Versatile Disc-Recordable (DVD+R/-R) recorded
Future Aspect-Import: The eCTD will be validated and imported into the THAI FDA Review System
Feedback: Application feedback (if there are problems experienced during the upload) and review of application by Thai FDA
Ensure that you do not use. 1. Double-sided discs 2. Re-writable disc (protection, authenticity and Stability of information cannot
Ensure that you do not use:
- Double-sided discs,
- Re-writable discs (protection, authenticity, and stability of information cannot be guaranteed)
- Compressed or zipped files (except for validation reports)
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
///////