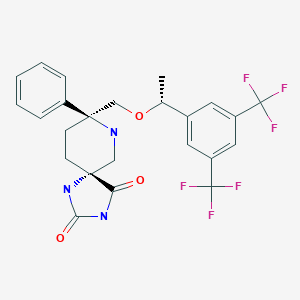
Telmapitant
TELMAPITANT; Telmapitant (USAN); Telmapitant [USAN]; 552292-58-7; HJ5FE4153B; D10391
(5R,8S)-8-[[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]-8-phenyl-1,3,9-triazaspiro[4.5]decane-2,4-dione
1,3,7-Triazaspiro[4.5]decane-2,4-dione, 8-[[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]-8-phenyl-, (5R,8S)-
(5R,8S)-8-(((1R)-1-(3,5-Bis(Trifluoromethyl)phenyl)ethoxy)methyl)-8-phenyl-1,3,7- triazaspiro(4.5)decane-2,4-dione
1,3,7-Triazaspiro(4.5)decane-2,4-dione,
8-(((1R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)methyl)-8-phenyl-, (5R,8S)-
Molecular Formula: C24H23F6N3O3
Molecular Weight: 515.448139
cas 552292-58-7
Merck & Co. (innovator)
Treatment of Nausea and Vomiting,
SYNTHESIS
……………………………………….
US7902366
http://www.google.com/patents/US7902366
Example 43a Example 43b
Step 1:
To a suspension of lactol Compound 3 (60 g, 93.0 mmol, 1 equiv.) and Wittig Reagent (93.5 g, 200.0 mmol, 2.15 equiv.) in toluene (800 ml) stirred at −78° C. under N2, a solution of KHMDS (0.5M in toluene, 558 ml, 280.0 mmol, 3 equiv.) was added dropwise at −78° C. The cooling bath was removed and the yellow mixture was warmed to RT to form a red solution. The mixture was allowed to stir at 23° C. for further 1 h before being quenched with saturated NH4Cl solution. EtOAc was added and layers were separated. The separated aqueous layer was extracted with EtOAc (2×500 ml). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [5% EtOAc-hexane to 10% EtOAc-hexane] gave alkene Compound 42 as white solid (40.5 g, 68%), Electrospray MS [M+1]+ 638.1. Continuous elution gave an impure cyclized product Compound 43.
Step 2:
A suspension of alkene Compound 42 (40.5 g, 64 mmol, 1 equiv.) and PtO2 (1.44 g, 6.4 mmol, 0.1 equiv.) in EtOH (400 ml) were stirred under a H2 balloon at 23° C. for 24 h. Another batch of PtO2 (1.44 g, 6.4 mmol, 0.1 equiv) was added and the mixture was stirred for another 24 h at 23° C. The catalyst was filtered via a pad of Celite. This solution of alkane Compound 44 was used in the next step without further purification.
Step 3:
p-TsOH.H2O (2.42 g, 13.0 mmol) was added to the ethanolic solution of alkane Compound 44 from above and the solution was heated to reflux for 4 h. The solution was cooled to RT and neutralized with Et3N. Solvents were removed in vacuum and EtOAc was added. Saturated NaHCO3 solution was added and layers were separated. The separated aqueous layer was extracted with EtOAc (300 ml×2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [10% ether-hexane] gave enamide Compound 45 (first batch) as yellow oil. Some intermediate and starting material were recovered as yellow oil by continuous elution with [50% EtOAc-hexane]. The yellow oil was dissolved in toluene and 10 mol % p-TsOH was added. The mixture was heated to reflux for 2 h and cooled to RT. Work up was as above and the combined enamide Compound 45 (25 g, 70%), Electrospray MS [M+1]+ 564.1, was obtained as yellow oil.
Step 4:
BH3.Me2S (13.6 ml, 133 mmo, 3.02 equiv) was added to a solution of enamide Compound 45 (25 g, 44.0 mmol,1 equiv.) in THF at 23° C. under N2. The mixture was stirred at 23° C. for 18 h and then cooled over an ice-water bath. A solution of NaOH (500 ml, 2N) was added slowly followed by a solution of H202 (500 ml, 30% aqueous). The mixture was allowed to stir from 0° C. to 23° C. for 18 h. Layers were separated and the separated aqueous layer was extracted with Et2O (500 ml×2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [hexane-EtOAc, 3:1 (v/v)] gave alcohol Compound 46 as colorless oil (19 g, 74%), Electrospray MS [M+1]+ 582.1.
Step 5:
Oxalyl chloride (5.7 ml, 65.3 mmol, 2 equiv.) was added to a solution of DMSO (9.3 ml, 131.0 mmol, 4 equiv.) in CH2Cl2 (300 ml) at −78° C. under N2. The mixture was stirred at −78° C. for 15 min before a solution of alcohol Compound 46 (19 g, 32.7 mmol. 1 equiv.) in CH2Cl2 (50 ml) was added. The mixture was stirred at −78° C. for a further 1 h and Et3N (32 ml, 228.9 mmol, 7 equiv.) was added. The cooling bath was removed and the mixture was warmed to RT before it was quenched with saturated NaHCO3 solution. Layers were separated and the aqueous was extracted with CH2Cl2 (300 ml×2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [hexane-ether, 4:1 (v/v)] gave ketone Compound 47 as colorless oil (15 g, 80%), Electrospray MS [M+1]+ 580.1.
Step 6:
EtOH (150 ml) was added to Cbz-ketone Compound 47 (15 g, 25.88 mmol, 1 equiv.), followed by NH4(CO3)2 (9.95 g, 103.5 mmol, 4 equiv.) and a solution of KCN (3.4 g, 51.77 mmol, 2 equiv.). The resulting mixture was heated at 58° C. under N2 for 72 h. TLC (1:1 EtOAc:hexane) revealed complete consumption of the starting material. The reaction mixture was cooled to RT and poured into sat. aq. NaHCO3 (200 ml) and extracted with EtOAc (3×200 ml). The combined organic layers were dried over MgSO4 and concentrated in vacuo to afford crude Cbz-hydantoin Compound 48 (16.5 g, 98%), Electrospray MS [M+1]+650.1. The crude material was used in the next reaction without further purification.
Step 7:
The crude Cbz-hydantoin Compound 48 (16.5 g, 25.4 mmol, 1 equiv.) was dissolved in MeOH (220 ml) and 20% Pd(OH)2—C (3.6 g) was added. The reaction mixture was shaken in a parr shaker under H2 atmosphere at 40 psi for 18 h. TLC (1:1 EtOAc:hexane) revealed complete consumption of the starting material. The reaction mixture was filtered through a pad of celite and the celite was washed with MeOH. The resulting solution was concentrated in vacuo. The crude product was purified by column chromatography on a Biotage (3:2, EtOAc:hex). Two major spots were collected. The less-polar spot corresponds to the isomer Example 43a (3 g, overall 20% over two steps), Electrospray MS [M+1]+ 516.1. The more polar spot corresponds to the isomer Example 43b (4.5 g, overall 30% over two steps), Electrospray MS [M+1]+ 516.1.
………………………………..
http://www.google.com/patents/WO2003051840A1?cl=en
Example 43a Example 43b
Step 1 :
Compound 3
To a suspension of lactol Compound 3 (60g, 93.0mmol, lequiv.) and Wittig Reagent (93. δg, 200.0mmol, 2.1 δequiv.) in toluene (800ml) stirred at -78°C under δ N2, a solution of KHMDS (O.δM in toluene, δδδml, 280.0mmol, 3equiv.) was added dropwise at -78°C. The cooling bath was removed and the yellow mixture was warmed to RT to form a red solution. The mixture was allowed to stir at 23°C for further 1 h before being quenched with saturated NH CI solution. EtOAc was added and layers were separated. The separated aqueous layer was extracted with EtOAc 0 (2 x δOOml). The combined organic layers were dried (MgSO ) and filtered.
Removal of solvents in vacuum followed by Biotage column chromatography [δ% EtOAc-hexane to 10% EtOAc-hexane] gave alkene Compound 42 as white solid (40. δg, 68%), Electrospray MS [M+1]+ 638.1. Continuous elution gave an impure cyclized product Compound 43. δ Step 2:
Compound 42
A suspension of alkene Compound 42 (40. δg, 64mmol, lequiv.) and PtO2 (1.44g, 6.4mmol, 0.1 equiv.) in EtOH (400ml) were stirred under a H2 balloon at 23°C for 24 h. Another batch of PtO2 (1.44g, 6.4mmol, 0.1 equiv) was added and the 0 mixture was stirred for another 24 h at 23°C. The catalyst was filtered via a pad of Celite. This solution of alkane Compound 44 was used in the next step without further purification. Step 3:
Compound 44
p-TsOH.H2O (2.42g, 13.0mmol) was added to the ethanolic solution of alkane
Compound 44 from above and the solution was heated to reflux for 4 h. The solution was cooled to RT and neutralized with Et3N. Solvents were removed in vacuum and EtOAc was added. Saturated NaHCO3 solution was added and layers
5 were separated. The separated aqueous layer was extracted with EtOAc (300ml x
2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [10% ether- hexane] gave enamide Compound 45 (first batch) as yellow oil. Some intermediate and starting material were recovered as yellow oil by continuous elution with 0 [50%EtOAc-hexane]. The yellow oil was dissolved in toluene and 10mol% p-TsOH was added. The mixture was heated to reflux for 2 h and cooled to RT. Work up was as above and the combined enamide Compound 45 (2δg, 70%), Electrospray
MS [M+1]+ 664.1 , was obtained as yellow oil.
Step 4:
BH3.Me2S (13.6ml, 133mmo, 3.02 equiv) was added to a solution of enamide Compound 45T25g, 44.0mmol, lequiv.) in THF at 23°C under N2. The mixture was stirred at 23°C for 18 h and then cooled over an ice-water bath. A solution of NaOH (600ml, 2N) was added slowly followed by a solution of H O2 (600ml, 30% 0 aqueous). The mixture was allowed to stir from 0°C to 23°C for 18 h. Layers were separated and the separated aqueous layer was extracted with Et.20 (600ml x 2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [hexane-EtOAc, 3:1 (v/v)] gave alcohol Compound 46 as colorless oil (19g, 74%), Electrospray MS [M+1]+ δ 582.1. Step 5:
Compound 46
Oxalyl chloride (δ.7ml, 6δ.3mmol, 2equiv.) was added to a solution of DMSO (9.3ml, 131.0mmol, 4equiv.) in CH2CI2 (300ml) at -78°C under N2. The mixture was 0 stirred at -78°C for 1 δ min before a solution of alcohol Compound 46 (19g, 32.7mmol. lequiv.) in CH2CI2 (50ml) was added. The mixture was stirred at -78°C for a further 1 h and Et3N (32ml, 228.9mmol, 7equiv.) was added. The cooling bath was removed and the mixture was warmed to RT before it was quenched with saturated NaHCO3 solution. Layers were separated and the aqueous was extracted with CH2CI2 (300ml x 2). The combined organic layers were dried (MgSO4) and filtered. Removal of solvents in vacuum followed by Biotage column chromatography [hexane-ether, 4:1 (v/v)] gave ketone Compound 47 as colorless oil (1δg, 80%), Electrospray MS [M+1]+ 680.1.
EtOH (150ml) was added to Cbz-ketone Compound 47 (15g, 2δ.88mmol, lequiv.), followed by NH (CO )2 (9.9δg, 103.5mmol, 4equiv.) and a solution of KCN (3.4g, 61.77mmoI, 2equiv.). The resulting mixture was heated at 68°C under N2 for 72 h. TLC (1 :1 EtOAc:hexane) revealed complete consumption of the starting
1δ material. The reaction mixture was cooled to RT and poured into sat. aq. NaHCO3 (200 ml) and extracted with EtOAc (3 x 200ml). The combined organic layers were dried over MgSO4 and concentrated in vacuo to afford crude Cbz-hydantoin Compound 48 (16.δg, 98%), Electrospray MS [M+1]+ 650.1. The crude material was used in the next reaction without further purification.
20 Step 7:
The crude Cbz-hydantoin Compound 48 (16.5g, 2δ.4mmol, lequiv.) was dissolved in MeOH (220ml) and 20% Pd(OH)2-C (3.6g) was added. The reaction mixture was shaken in a parr shaker under H2 atmosphere at 40 psi for 18 h. TLC (1 :1 EtOAc:hexane) revealed complete consumption of the starting material. The
26 reaction mixture was filtered through a pad of celite and the celite was washed with MeOH. The resulting solution was concentrated in vacuo. The crude product was purified by column chromatography on a Biotage (3:2, EtOAc:hex). Two major spots were collected. The less-polar spot corresponds to the isomer Example 43a (3 g, overall 20% over two steps), Electrospray MS [M+1]+ 616.1. The more polar spot
30 corresponds to the isomer Example 43b (4.6 g, overall 30% over two steps), Electrospray MS [M+1]+ 616.1.
|
4-29-2011
|
NK1 ANTAGONISTS
|
|
|
3-9-2011
|
NK1 antagonists
|
| English translation of Knabe, J., et al., “Racemates and Enantiomers of . . . ,” Pharmazie 52(12):912-919 (1997). | ||
| 2 | English translation of Schult, Karl E., et al., “Hydantoin-Derivate as Potential . . . ,” Eur. J. Med. Chem.-Chimica Therapeutics 13(1):25-31 (1978). | |
| 3 | English translation of Schult, Karl E., et al., “Hydantoin-Derivate as Potential . . . ,” Eur. J. Med. Chem.—Chimica Therapeutics 13(1):25-31 (1978). | |
| 4 | Knabe, J., et al., “Racemates and Enantiomers of Basic Substituted 5-Phenylhydantoins . . . ,” Pharmazie 52(12): 912-919 (1997). | |
| 5 | Oh, Chang-Hyun et al., “Synthesis of New Hydantoin-3-Acetic Acid Derivatives . . . ,” Bull. Korean Chem. Soc. 9(4):231-235 (1988). | |
| 6 | Shulte, Karl E., et al., “Hydantoin-Derivate als . . . ,” Eur. J. Med. Chem.-Chimica Therapeutica 13(1):25-31 (1978). | |
| 7 | Shulte, Karl E., et al., “Hydantoin-Derivate als . . . ,” Eur. J. Med. Chem.—Chimica Therapeutica 13(1):25-31 (1978). | |
| 8 | Wu, X. et al., “Generation of Cyclopenta [c] piperidines and Pyrrolo [3,4-c]piperidines- . . . ,” Tetrahedron 56(34): 6279-6290 (2000). | |
| 9 | * | Xiujuan Wu et al 2000. , Stereoselective transformation of 2H-1,4-Oxazin-2-ones into 2,(2),5,5-tri- and tetrasubstituted Analogues. . . |
| US6436928 * | Dec 14, 2000 | Aug 20, 2002 | Schering Corporation | Selective neurokinin antagonists |
| US6635639 * | Feb 13, 2002 | Oct 21, 2003 | Nps Allelix Corp. | Use of N-alkylamino-heterocylic compounds for the treatment of migraine |
| US7041682 * | Jul 2, 2003 | May 9, 2006 | Schering Corporation | Antiemetics, antidepressants, anxiolytic agents, antitussive agents |
| US7122677 * | Nov 12, 2002 | Oct 17, 2006 | Scherig Corporation | NK1 antagonists |
| US20060094720 * | Dec 15, 2005 | May 4, 2006 | Neng-Yang Shih | NK1 antagonists |
| US20060223804 * | Jun 30, 2005 | Oct 5, 2006 | Schering Corporation | NK1 antagonists |
| EP0790248A1 | Jan 20, 1997 | Aug 20, 1997 | Pfizer Limited | 3-Aza-piperidone- (tetrahydropyrimidin-2-one) and 3-oxa-piperidone (1,3 oxazin-2-one) derivatives, their preparation and their use as tachykinin/neurokinin antagonists |
key
Telmapitant, Merck, Tachykinin NK1 Antagonists