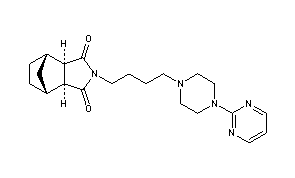
Tandospirone, [112457-95-1]
US 5011841
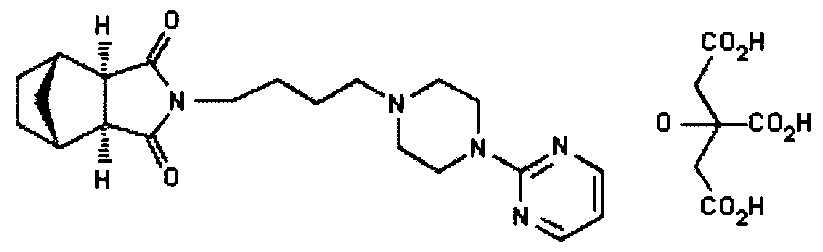
(lR*,2S*,3R*,4S*)-N-[4-[4-(2- US 5011841 citrate Pyrimidinyl) piperazin-1-
yl] butyl ] -2 , 3-norbornane- dicarboximide citrate
Tandospirone hyd, SM-3997,
Chemical Name: (3aR,4S,7R,7aS)-rel-Hexahydro-2-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-4,7-methano-1H-isoindole-1,3(2H)-dione hydrochloride
Tandospirone (Sediel), also known as metanopirone, is an anxiolytic andantidepressant used in China and Japan, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone and piperazine chemical classes and is closely related to other agents like buspirone and gepirone.
Tandospirone is most commonly used as a treatment for anxiety and depressive disorders, such as generalised anxiety disorder and dysthymia respectively.[1] For both indications it usually takes a couple of weeks for therapeutic effects to be start being seen,[1] although at higher doses more rapid anxiolytic responses have been seen.[2] It has also been used successfully as a treatment for bruxism.[3]
Tandospirone has also been tried, successfully, as an adjunctive treatment for cognitive symptoms in schizophrenic individuals.[4]
It is not believed to be addictive but it is known to produce mild withdrawal effects (e.g. anorexia) after abrupt discontinuation.[1]
Chemistry
Yevich, Joseph P.; New, James S.; Smith, David W.; Lobeck, Walter G.; Catt, John D.; Minielli, Joseph L.; Eison, Michael S.; Taylor, Duncan P. et al. (1986). “Synthesis and biological evaluation of 1-(1,2-benzisothiazol-3-yl)- and (1,2-benzisoxazol-3-yl)piperazine derivatives as potential antipsychotic agents”. Journal of Medicinal Chemistry 29 (3): 359–69. doi:10.1021/jm00153a010.PMID 2869146.
Tandospirone acts as a potent and selective 5-HT1A receptor partial agonist, with a Ki affinity value of 27 ± 5 nM[5] and approximately 55-85% intrinsic activity.[6][7] It has weak and clinically negligible affinity for the 5-HT2A (1,300 ± 200), 5-HT2C (2,600 ± 60), α1-adrenergic (1,600 ± 80), α2-adrenergic (1,900 ± 400), D1 (41,000 ± 10,000), and D2 (1,700 ± 300) receptors, and is essentially inactive at the 5-HT1B, 5-HT1D, β-adrenergic, and muscarinic acetylcholine receptors, serotonin transporter (SERT), and benzodiazepine (BDZ)allosteric site of the GABAA receptor (all of which are > 100,000).[5] There is evidence of tandospirone having low but significantantagonistic activity at the α2-adrenergic receptor through its active metabolite 1-(2-pyrimidinyl)piperazine (1-PP), however.[8][9]
- Barradell, LB; Fitton, A (February 1996). “Tandospirone” (PDF). CNS Drugs 5 (2): 147–153. doi:10.2165/00023210-199605020-00006.
- Nishitsuji; To, H; Murakami, Y; Kodama, K; Kobayashi, D; Yamada, T; Kubo, C; Mine, K (2004). “Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression : results of a comparatively high dosage trial” (PDF). Clinical drug investigation 24 (2): 121–6. doi:10.2165/00044011-200424020-00007. PMID 17516698.
- “Tandospirone”. Martindale: The Complete Drug Reference (The Royal Pharmaceutical Society of Great Britain). 23 September 2011. Retrieved 14 November 2013.
- Sumiyoshi, T; Matsui, M; Nohara, S; Yamashita, I; Kurachi, M; Sumiyoshi, C; Jayathilake, K; Meltzer, HY (October 2001). “Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment” (PDF). The American Journal of Psychiatry 158 (10): 1722–1725. doi:10.1176/appi.ajp.158.10.1722. PMID 11579010.
- Hamik; Oksenberg, D; Fischette, C; Peroutka, SJ (1990). “Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites”. Biological Psychiatry 28 (2): 99–109. doi:10.1016/0006-3223(90)90627-E. PMID 1974152.
- Tanaka; Tatsuno, T; Shimizu, H; Hirose, A; Kumasaka, Y; Nakamura, M (1995). “Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain”. General pharmacology 26 (8): 1765–72. doi:10.1016/0306-3623(95)00077-1.PMID 8745167.
- Yabuuchi, Kazuki; Tagashira, Rie; Ohno, Yukihiro (2004). “Effects of tandospirone, a novel anxiolytic agent, on human 5-HT1A receptors expressed in Chinese hamster ovary cells (CHO cells)”. Biogenic Amines 18 (3): 319. doi:10.1163/1569391041501933.
- Blier; Curet, O; Chaput, Y; De Montigny, C (1991). “Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine–II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission”. Neuropharmacology 30 (7): 691–701. doi:10.1016/0028-3908(91)90176-C. PMID 1681447.
- Miller; Thompson, ML; Byrnes, JJ; Greenblatt, DJ; Shemer, A (1992). “Kinetics, brain uptake, and receptor binding of tandospirone and its metabolite 1-(2-pyrimidinyl)-piperazine”. Journal of Clinical Psychopharmacology 12 (5): 341–5. PMID 1362206
-
“Azapirone” is a term that has been used to describe a structural class of psychotropic compounds that demonstrate similar pharmacology relating to interaction with monoaminergic pathways in particular brain regions.
-
[0002]
-
[0003]
-
[0004]Perhaps the best known representative of the azapirone class of psychotropic agents is buspirone (1), originally disclosed in U.S. 3,71 7,634.
-
[0005]
-
[0006]
-
[0007]The dotted and solid lines in the tandospirone-type structure can be taken as either a single or double carbon-carbon covalent bond.
-
[0008]While a number of synthetic processes have been disclosed for the synthesis of these azapirones, a method of choice, currently used for large scale preparation of buspirone and gepirone, was disclosed by Sims in U.S. 4,351,939. The Sims method involves the reaction of an appropriately-substituted glutarimide (3) with a novel spiroquatemary ammonium halide (4) to yield buspirone or gepirone or
close analogs. The halide, X, is preferably bromide. The reaction is carried out in a hot inert reaction medium in the presence of an acid scavenging base. In practice, the reaction process involves a multiphasic reaction of (3) and (4) in refluxing xylene with an excess of solid potassium carbonate.
-
[0009]For large-scale production, this prior art synthesis suffers from several processing disadvantages, including:
- · high temperature processing in toxic solvents, e.g., refluxing xylene;
- · a multiphasic reaction mixture requiring highly efficient stirring and as the scale increases, this factor becomes increasingly important;
- • the presence of large amounts of inorganic by-products which complicate reaction workup and product isolation;
- • long reaction time, e.g., 24 hours; and
- • lower and more erratic yields of product resulting from the generation of water as a by-product. The efficient removal of the water is a problem, particularly in these large-scale processes.
-
[0010]Compounds of formula (2)
such as imidate anions of structure (2a),
wherein MIB represents an alkali or alkaline earth metal, can be reacted with a pyrimidinylpiperazinyl derivative of formula (5),
wherein Q is a nucleofuge, i.e. a leaving group of the type commonly utilized in synthetic organic chemistry; by heating in an inert solvent under standard conditions such as those described for the alkylation step of the Gabriel synthesis; cf: Gibson and Bradshaw, Angew. Chem. Int. Ed., 7/919,930, (1968).
-
[0011]The reaction of certain anions, e.g., (2) with intermediates of formula (5), has been previously disclosed. This method has been reported, for example, for the preparation of buspirone (U.S. 3,717,634) and ipsapirone (U.S. 4,818,756). In general, this method has not been used on a large scale, particularly with imides, due to the additional processing requirements necessitated by the generation and handling of a reactive metal salt of the imidate component. As mentioned, the Sims process (involving the reaction of (3) and (4)-type compounds) is the current method of choice for large-scale synthesis of these azapirones.
-
[0012]These prior art processes differ then from the novel improved process which utilizes a pre-formed potassium salt of the imidate-type starting material (2) which is reacted with a spiroquatemary salt (4), instead of a formula (5) compound, to provide azapirone product
Tandospirone and tandospirone salts have been described in several patents and patent applications. These describe pharmaceutical compositions of tandospironealone and in combination with other drugs for treatment of human disease and include EP 0437026 (Treatment of depression), WO 1994016699 (Compositions containing tandospirone or its analogues), EP 0082402 (Succinimide derivates and process for preparation thereof), JP 2002020291 (Therapeutic agents for cognition disorders), JP 2003335678 (Therapeutic agents for neurogenic pain), WO 2004002487 (Methods for treating attention deficit disorder), JP 2005225844 (Agents for the treatment of irritable bowel syndrome), WO 20051 17886 (Adhesive patch),WO 2008044336 (Crystal- containing adhesive preparation) and WO 2010065730 (Pharmaceutical suspension).
………………………………………
Drugs Fut 1986,11(11),949
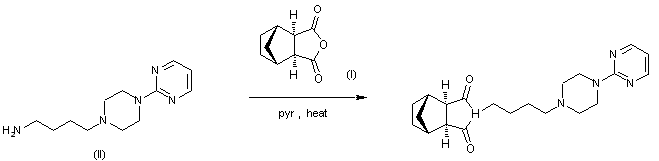
By condensation of norbornane-2,3-di-endo-carboxylic anhydride (I) with 1-(4-aminobutyl)-4-(2 pyrimidinyl)piperazine (II) in refluxing pyridine.
……………………………………………………….
CA 1184915; EP 0082402
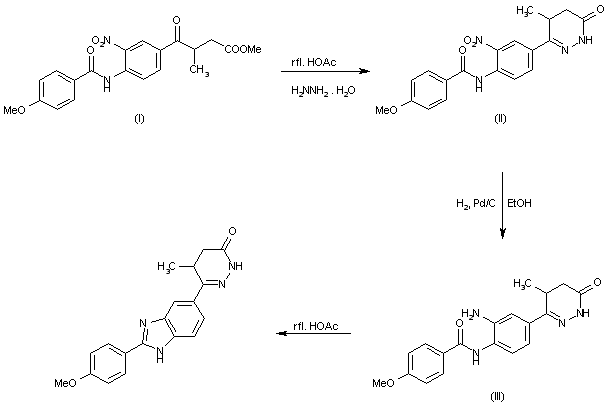
The cyclization of methyl 3-[4-(4-methoxybenzoylamino)-3-nitrobenzoyl]butyrate (I) with hydrazine hydrate in refluxing acetic acid gives 4,5-dihydro-6-[4-(4-methoxybenzoylamino)-3-nitrobenzoyl]-5-methylpyridazin-3(2H)-one (II), which is reduced with H2 over Pd/C in ethanol yielding the corresponding amino derivative (III). Finally, this compound is cyclized in refluxing acetic acid.
……………………………………………………..
Chem Pharm Bull 1991,39(9),2288
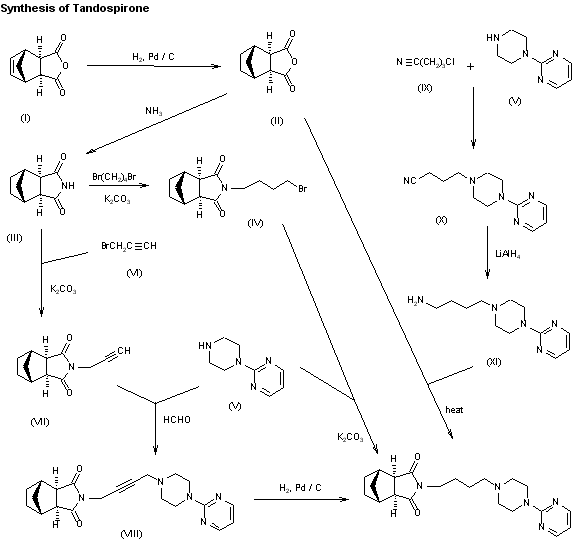
New syntheses of tandospirone have been described: 1) The hydrogenation of bicyclo[2.2.1]hept-5-ene-2,3-di-exo-carboxylic acid anhydride (I) with H2 over Pd/C in THF-water gives bicyclo[2.2.1]heptane-2,3-di-exo-carboxylic acid anhydride (II), which by reaction with ammonia in THF-water is converted into the imide (III). The reaction of (III) with 1,4-dibromobutane by means of K2CO3 in refluxing acetone yields N-(4-bromobutyl)bicyclo[2.2.1]heptane-2,3-di-exo-carboximide (IV), which is finally condensed with 1-(2-pyrimidinyl)piperazine (V) by means of K2CO3 and KI in hot DMF. 2) Imide (III) is condensed with propargyl bromide (VI) by means of K2CO3 in refluxing acetone affording N-propargylbicyclo[2.2.1]heptane-2,3-di-exo-carboximide (VII), which is allowed to react with piperazine (V) and formaldehyde by means of CuSO4 in dioxane to give N-[4-[4-(2-pyrimidinyl)piperazin-1-yl]-2-butynyl]bicyclo[2.2.1]heptane-2,3-di-exo-carboximide (VIII). Finally, this compound is reduced with H2 over Pd/C. 3) The condensation of piperazine (V) with 4-chlorobutyronitrile (IX) by means of NaOH in acetone gives 4-[4-(2-pyrimidinyl)piperazin-1-yl]butyronitrile (X), which is reduced with LiAlH4 in ether yielding 1-(4-aminobutyl)-4-(2-pyrimidinyl)piperazine (XI). Finally, this compound is condensed with anhydride (II) in refluxing pyridine.
………………….



…………