Example 1: preparation of 2-fluoropropanoyl chloride (3)
Chlorosulfonic acid (660 mL, 10 mol, 20 eq) was added to a solution of phthaloyl dichloride (1.4 L, 10 mol, 20 eq) and ethyl-2-fluoropropanoate (600 g, 5 mol) at room temperature. The solution was heated at 120 ℃ for 4 hs. 2- (R) -fluoropropanoyl chloride was distilled from the reaction mixture under reduced pressure and recovered as a colourless oil (320 g, 58.2%) . 1H-NMR (CDCl3, 400 MHz) : δ 5.08 (dq, J = 48.8, 6.8 Hz, 1 H) , 1.63 (dd, J =22.8, 6.8 Hz, 3 H) .
Example 2: preparation of (4R) -3- (2-fluoropropanoyl) -4-isopropyloxazolidin-2-one (4)
n-Butyl lithium (2.5 M in hexane, 30 mL, 75 mmol, 1.1 eq) was added to a solution of 4-(R) -4-isopropyl-2-oxazolidinone (8.8 g, 68.2 mmol, 1 eq) in dry THF (80 mL) at -50 ℃ under N2 atomosphere. After 30 min, 2-fuoropropanoyl chloride (6.8 mL, 0.9 eq) was added, and the solution was stirred for 4 hs at -50 ℃. The reaction was then quenched with a saturated solution of NH4Cl (50 mL) , extracted with MTBE (80 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure. The product was purified over silica (Hexane/EtOAc= 10/1) and recovered as a brown oil (9 g, 74.8%) . 1H-NMR (CDCl3, 400 MHz) : δ 6.00 (dm, J = 49.2Hz, 1 H) , 4.27 -4.53 (m, 3 H) , 2.43 (dm, J = 52.6 Hz, 1 H) , 1.63 (td, J = 23.2Hz, 3 H) , 0.92 (dq, J = 17.8 Hz, 6 H) .
[0206]
Example 3: preparation of (4S) -3- (2-fluoropropanoyl) -4-isopropyloxazolidin-2-one (5)
n-Butyl lithium (2.5 M in hexane, 75 mL, 187 mmol, 1.1eq) was added to a solution of 4- (S) -4-isopropyl-2-oxazolidinone (22 g, 170 mmol, 1 eq) in dry THF (200 mL) at -50 ℃ under N2 atomosphere. After 30 min 2-fuoropropanoyl chloride (17 mL, 153 mmol, 0.9 eq) was added, and the solution was stirred for 1 h at -50 ℃. After the starting material was completely consumed, the reaction was then quenched with a saturated solution of NH4Cl (125 mL) , extracted with MTBE (200 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure. The product was purified over silica (hexane/EtOAc= 10/1) and recovered as a brown oil (34 g, 83.3%) . 1H-NMR (CDCl3, 400 MHz) : δ 5.93 (dm, J = 48.8 Hz, 1 H) , 4.19 -4.17 (m, 3H) , 2.35 (dm, J = 52.8 Hz , 1 H) , 1.55 (td, J = 23.6 Hz, 3 H) , 0.85 (dq, J = 18 Hz, 6 H) .
Example 4: preparation of (4R) -3- (2-fluoropropanoyl) -4-phenyloxazolidin-2-one (6)
n-Butyl lithium (2.5 M in hexane, 13.5 mL, 33.74 mmol, 1.1 eq) was added to a solution of (R) -4-phenyloxazolidin-2-one (5 g, 30.67 mmol, 1 eq) in dry THF (75 mL) at -50 ℃ under N2 atomosphere. After 30 minutes, 2-fuoropropanoyl chloride (3.75 g, 33.74 mmol) was added, and the solution was stirred for 1 h at -50 ℃ to -60 ℃. The reaction was then quenched with a saturated solution of NH4Cl, extracted with EtOAc, washed with NaHCO3(sat) , brine and dried over MgSO4. Solvents were removed under reduced pressure. The product was purified over silica (hexane /EtOAc) and recovered as a brown oil (4 g, 55%) . 1H-NMR (CDCl3, 400 MHz) : δ 7.35-7.21 (m, 5 H) , 5.99-5.84 (md, 1 H) , 5.42-5.33 (dd, 1 H) , 4.72 (dd, 1 H) , 4.31 (m, 1 H) , 1.50 (m, 3 H) .
Example 5: preparation of (4s) -3- (2-fluoropropanoyl) -4-phenyloxazolidin-2-one (7)
n-Butyl lithium (2.5 M in hexane, 67.5 mL, 169 mmol, 1.1 eq) was added to a solution of (s) -4-phenyloxazolidin-2-one (25 g, 153 mmol, 1 eq) in dry THF (375 mL) at -60 ℃ under N2 atomosphere. After 30 min, 2-fuoropropanoyl chloride (18.7 g, 169 mmol) was added, and the solution was stirred for 1h at -50 ℃ to -60 ℃. The reaction was then quenched with a saturated solution of NH4Cl, extracted with EtOAc, washed with NaHCO3 (sat) , brine and dried over MgSO4. Solvents were removed under reduced pressure. The product was purified over silica (hexane /EtOAc) and recovered as a brown oil (16.5 g, 45.4%) . 1H-NMR (CDCl3, 400 MHz) : δ 7.36-7.20 (m, 5 H) , 5.95-5.80 (md, 1 H) , 5.42-5.30 (dd, 1 H) , 4.71 (dd, 1 H) , 4.30 (m, 1 H) , 1.51 (m, 3 H) .
Example 6: preparation of (4S) -4-benzyl-3- (2-fluoropropanoyl) oxazolidin-2-one (8)
n-Butyl lithium (2.5 M in hexane, 54.7 mL, 137 mmol, 1.1eq) was added to a solution of (S) -4-benzyloxazolidin-2-one (22 g, 124 mmol, 1eq) in dry THF (220 mL) at -60 ℃ under N2 atomosphere. After stirring 30 min at -60 ℃, 2-fuoropropanoyl chloride (15.2 g, 137 mmol) was added dropwisely below -50 ℃ , after adding the solution was stirred for 1h at -50 ℃ to -60 ℃. The reaction was then quenched with a saturated solution of NH4Cl, extracted with EtOAc, washed with NaHCO3 (sat) , brine and dried over MgSO4. Solvents were removed under reduced pressure. The product was purified over silica (hexane/EtOAc) and recovered as a brown oil (25.8 g, 82.7%) . 1H-NMR(400 MHz, CDCl3 ) : δ 7.29-7.13 (m, 5 H) , 6.01-5.81 (qd, 1 H) , 4.71-4.58 (md, 1 H) , 4.29-4.04 (m, 2 H) , 3.32-3.16 (dd, 1 H) , 2.79-2.74 (m, 1 H) , 1.51 (m, 3 H) .
Example 7: preparation of (4R) -4-benzyl-3- (2-fluoropropanoyl) oxazolidin-2-one (9)
Use the procedure described in Example 6, (R) -4-benzyloxazolidin-2-one as the start material to give the desired compound (4R) -4-benzyl-3- (2-fluoropropanoyl) oxazolidin-2-one (yield: 85%) . 1H-NMR (400 MHz, CDCl3 ) : δ 7.27 -7.12 (m, 5 H) , 6.00-5.83 (qd, 1 H) , 4.72-4.55 (md, 1 H) , 4.27-4.03 (m, 2 H) , 3.32 -3.16 (dd, 1 H) , 2.79 -2.72 (m, 1 H) , 1.53 (m, 3 H) .
[0221]
Example 8: preparation of (4R) -3- (2-fluoropropanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (10)
[0223]
n-Butyl lithium (2.5 M in hexane, 48 mL) was added to a solution of (R) -4-isopropyl-5,5-diphenyloxazolidin-2-one (28.1 g) in dry THF (150 mL) at -65 ℃ under N2 atomosphere. After stirring 30 min at -60 ℃, 2-fuoropropanoyl chloride (16.4 g, 1.5 eq) was added dropwisely below -60 ℃. After adding the solution was stirred for 2 h at -60 ℃. The reaction was then quenched with a saturated solution of NH4Cl, extracted with EtOAc, washed with NaHCO3 (sat) , brine and dried over MgSO4. Solvents were removed under reduced pressure. The crude product was recrystalized in (DCM/PE) to give (4R) -3- (2-fluoropropanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (30 g, 85%) . 1H-NMR (CDCl3, 400 MHz) : δ 7.50 -7.26 (m, 10 H) , 5.89 (ddq, J = 64.4, 49.3, 6.6 Hz, 1 H) , 5.37 (dd, J = 70.8, 3.4 Hz, 1 H) , 2.00 (dd, J = 7.3, 3.3 Hz, 1 H) , 1.70 (dd, J = 23.4, 6.7 Hz, 1.5 H) , 1.12 (dd, J = 23.8, 6.6 Hz, 1.5 H) , 0.83 (ddd, J = 28.0, 16.7, 6.9 Hz, 6 H) .
[0224]
Example 9: preparation of (4S) -3- (2-fluoropropanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (11)
[0226]
Use the procedure described in Example 8 and (S) -4-isopropyl-5, 5-diphenyloxazolidin-2-one as the start material to give the desired compound (4S) -3- (2-fluoropropanoyl) -4-isopropyl- 5,5-diphenyl oxazolidin-2-one (yield: 82%) . 1H-NMR (CDCl3, 400 MHz) : δ 7.51 -7.27 (m, 10 H) , 5.90 (ddq, J = 64.4, 49.3, 6.6 Hz, 1 H) , 5.38 (dd, J = 70.8, 3.4 Hz, 1H) , 2.01 (dd, J = 7.3, 3.3 Hz, 1 H) , 1.71 (dd, J = 23.4, 6.7 Hz, 1.5 H) , 1.13 (dd, J = 23.8, 6.6 Hz, 1.5 H) , 0.84 (ddd, J = 28.0, 16.7, 6.9 Hz, 6 H) .
[0227]
Example 10: preparation of (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (12)
[0229]
Method A: TiCl4 (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4R) -3- (2-fluoropropanoy l ) -4-isopropyloxazolidin-2-one (4) (10 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, diisopropylethyl amine (10.3 mL, 1.26 eq) was added and the solution was stirred for 2 hs at-78 ℃, then the second batch of TiCl4 (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added. After 10 min, acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -78 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into DCM (20 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (10.2 g, yield: 80%, purity: 97.2%) . 1H-NMR (400 MHz, CDCl3) : δ 5.89 (dddd, J = 17.1, 10.5, 6.5, 0.8 Hz, 1 H) , 5.42 (d, J =17.2 Hz, 1 H) , 5.30 (d, J = 10.1 Hz, 1 H) , 4.68 (dd, J = 14.8, 6.5 Hz, 1 H) , 4.44 (d, J = 4.0 Hz, 1 H) , 4.32 (t, J = 8.5 Hz, 1 H) , 4.24 (dd, J = 9.1, 3.4 Hz, 1 H) , 3.61 (d, J = 6.5 Hz, 1 H) , 2.37 (dd, J = 7.0, 4.1 Hz, 1 H) , 1.73 (s, 1.5 H) , 1.67 (s, 1.5 H) , 0.92 (ddd, J = 7.8, 5.6, 2.4 Hz, 6 H) ; 19F-NMR (400 MHz, CDCl3) : -158.3 ppm.
[0230]
Method B: TiCl4 (1 M in DCM, 50 mL, 50mmol, 1.1 eq) was added to a solution of (4R) -3- (2-fluoropropanoy l ) -4-isopropyloxazolidin-2-one (10 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, (-) -spartein (14.5 g, 1.26 eq) was added and the solution was stirred for 2 hs at-78 ℃, then the second batch of TiCl4 (1 M in DCM, 50 mL, 50 mmol, 1.1eq) was added. After 10 min, acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -78 ℃. Then the reaction was quenched with NH4Cl (sat 50 mL) . The products were extracted into DCM (20 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (9.4 g, yield: 75%, purity: 96.5%) .
[0231]
Example 11: preparation of (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (13)
[0233]
TiCl4 (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoropropanoy l ) -4-isopropyloxazolidin-2-one (4) (10 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, diisopropylethyl amine (15.9 g, 2.5 eq) was added and the solution was stirred for 2 hs at-78 ℃. Then acrylaldehyde (7 mL, 2eq) was added and the solution was stirred for 1 h at -78 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into DCM (20 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (10.4 g, yield: 83%, purity: 92.8%) . 1H-NMR (400 MHz, CDCl3) : δ 5.92 (d, J = 1.1 Hz, 1 H) , 5.44 (d, J = 17.2 Hz, 1 H) , 5.34 -5.28 (m, 1 H) , 4.73 (dd, J = 13.9, 6.2 Hz, 1 H) , 4.43 (m, 1 H) , 4.37 -4.30 (m, 1H) , 4.27 -4.21 (m, 1 H) , 2.43 -2.31 (m, 1H) , 1.77 (s, 1.5 H) , 1.71 (s, 1.5 H) , 0.91 (dd, J = 12.1, 7.0 Hz, 6 H) ; 19F-NMR (400 MHz, CDCl3) : δ -159.1ppm.
[0234]
Example 12: preparation of (S) -4-benzyl-3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) oxazolidin-2-one
[0236]
TiCl4 (1 M in DCM, 50 mL, 50mmol, 1.1 eq) was added to a solution of (4S) -4-benzyl-3-(2-fluoro propanoyl) oxazolidin-2-one (8) (12.3 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, TMEDA (15.9 g, 2.5 eq) was added and the solution was stirred for 2 hs at -78 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -78 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into DCM (20 mL*2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (13 g, yield: 86%, purity: 91.5%) . 1H-NMR (400 MHz, CDCl3) : δ 7.38 -7.27 (m, 3 H) , 7.22 (d, J = 6.8 Hz, 2 H) , 5.96 (dddd, J = 17.0, 10.5, 6.2, 1.2 Hz, 1 H) , 5.47 (d, J = 17.2 Hz, 1 H) , 5.35 (d, J = 10.5 Hz, 1 H) , 4.75 (dd, J = 13.9, 6.2 Hz, 1 H) , 4.66 (td, J = 7.1, 3.6 Hz, 1 H) , 4.23 (dd, J = 16.3, 5.0 Hz, 2 H) , 3.33 (dd, J = 13.3, 3.3 Hz, 1 H) , 2.76 (dd, J =13.3, 10.0 Hz, 1 H) , 1.81 (s, 1.5 H) , 1.76 (s, 1.5 H) ; 19F-NMR (400 MHz, CDCl3) : δ -158.47 ppm.
[0237]
Example 13: preparation of (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-phenyloxazolidin-2-one
[0239]
TiCl4 (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoropropanoyl) -4-phenyloxazolidin-2-one (7) (11.6 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, Et3N (12.5 g, 2.5 eq) was added and the solution was stirred for 2 hs at-78 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -78 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into DCM (20 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (12 g, yield: 83%, purity: 90.5%) . 1H-NMR (400 MHz, CDCl3) : δ 7.43 -7.30 (m, 5 H) , 5.81 (dddd, J = 17.0, 10.5, 6.3, 1.1 Hz, 1 H) , 5.46 (dd, J = 8.4, 5.1 Hz, 1 H) , 5.37 (dt, J = 17.2, 1.2 Hz, 1 H) , 5.23 (d, J = 10.4 Hz, 1 H) , 4.74 (t, J = 8.7 Hz, 1 H) , 4.64 (dd, J = 13.5, 6.3 Hz, 1 H) , 4.31 (dd, J = 8.9, 5.2 Hz, 1 H) , 1.60 (s, 1.5H) , 1.55 (s, 1.5 H) ; 19F-NMR (400 MHz, CDCl3) : δ -158.47 ppm.
[0240]
Example 14: preparation of (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-phenyloxazolidin-2-one
[0242]
TiCl4 (1 M in DCM, 50 mL, 50mmol, 1.1 eq) was added to a solution of (4R) -3- (2-fluoro propan oyl) -4-phenyloxazolidin-2-one (6) (11.6 g, 49.2 mmol, 1 eq) in dry DCM (170 mL) at -78 ℃ under N2 atomosphere. After 10 min, DIPEA (15.9 g, 2.5 eq) was added and the solution was stirred for 2 hs at-78 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -78℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into DCM (20 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the product was recrystalized in toluene to give the desired compound as a white solid (11.1 g, yield: 77%, purity: 91.5%) . 1H-NMR (400 MHz, CDCl3) : δ 7.44 -7.29 (m, 5 H) , 5.74 -5.63 (m, 1 H) , 5.48 (dd, J = 8.4, 5.3 Hz, 1 H) , 5.35 -5.26 (m, 1 H) , 5.15 (d, J = 10.5 Hz, 1 H) , 4.73 (t, 1 H) , 4.52 (dd, J = 14.8, 6.2 Hz, 1 H) , 4.28 (dd, J = 8.9, 5.3 Hz, 1 H) , 1.68 (s, 1.5 H) , 1.63 (s, 1.5 H) ; 19F-NMR (400 MHz, CDCl3) : δ -161.93 ppm.
[0243]
Example 15: preparation of (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one
[0245]
Method 1: LiHMDS (1 M in THF, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoro propanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (11) (17.4 g, 49.2 mmol, 1 eq) in dry THF (100 mL) at -20 ℃ under N2 atomosphere. After 1.5 hs, acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at -20 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (50 mL) . The products were extracted into EA (50 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the crude product was used directly in the next step. m/z (ES+) : 412 [M+H] +.
[0246]
Method 2: (n-Bu) 2BOTf (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoro propanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (11) (17.4 g, 49.2 mmol, 1 eq) in dry DCM (100 mL) at 0 ℃ under N2 atomosphere. After 15 min, 2, 6-lutidine (10.5g, 2eq) was added and the solution was stirred for 2 hs at 0 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at 0 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (100 mL) . The products were extracted into DCM (40 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the crude product was used directly in the next step (17.82 g, yield: 88% (Internal standard yield) .
[0247]
Method 3: (n-Bu) 2BOTf (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoro propanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (11) (17.4 g, 49.2 mmol, 1 eq) in dry DCM (100 mL) at 0 ℃ under N2 atomosphere. After 15 min, DIPEA (13 g, 2 eq) was added and the solution was stirred for 2 hs at 0 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at 0 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (100 mL) . The products were extracted into EA (50 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the crude product was used directly in the next step (16.2 g, yield: 80% (Internal standard yield ) .
[0248]
Method 4: (C6H12) 2BOTf (1 M in DCM, 50 mL, 50 mmol, 1.1 eq) was added to a solution of (4S) -3- (2-fluoro propanoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (11) (17.4 g, 49.2 mmol, 1 eq) in dry DCM (100 mL) at 0 ℃ under N2 atomosphere. After 15 min, 2, 6-lutidine (10.5 g, 2 eq) was added and the solution was stirred for 2 hs at 0 ℃. Then acrylaldehyde (7 mL, 2 eq) was added and the solution was stirred for 1 h at 0 ℃. Then the reaction was quenched with a saturated solution of NH4Cl (100 mL) . The products were extracted into DCM (50 mL *2) , washed with brine and dried over MgSO4. Solvents were removed under reduced pressure and the crude product was used directly in the next step (14.6 g, yield: 80% (Internal standard yield ) .
[0249]
Example 16: preparation of (3R, 4R, 5R) -3-fluoro-4-hydroxy-5- (hydroxymethyl) -3-methyl dihydro furan-2 (3H) -one
[0252]
N-Bromosuccinimide (19.6 g, 1.1 eq) was added portionwisely to a solution of (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (12) (25.9 g, 100 mmol, 1 eq) in DME/H2O (4: 1, 130ml) at -5 ℃, and stirred for 2 hs . After the reaction was complete, NaHCO3 (sat, 20 mL) was added and stirred for 0.5 h at rt. The mixture were extracted by DCM (50 mL *2) , washed with brine and dried over MgSO4. Solvents were removed, the residue dissolved by MTBE (1V) , the solid was filtered off to recover the auxiliary, the filtrate was concentrated to dryness to obtained the (3R, 4R, 5R) -5- (bromomethyl) -3-fluoro-4-hydroxy-3-methyldihydrofuran-2 (3H) -one (18a) . 1H-NMR (400 MHz, CDCl3) : δ 4.62 -4.53 (m, 1 H) , 4.37 (dd, J = 3.0, 1.9 Hz, 1 H) , 3.73 (dd, J = 10.1, 8.7 Hz, 1 H) , 3.60 (ddd, J = 10.1, 5.8, 1.9 Hz, 1 H) , 2.59 (dd, J = 2.5, 1.7 Hz, 1 H) , 1.67 (d, J = 22.7 Hz, 3 H) ; 19F-NMR (400 MHz, CDCl3) : δ -172.248 ppm.
[0253]
Alternative Method 1a: Br2 (17.6 g, 1.1 eq) was added portionwisely to a solution of (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (12) (25.9 g, 100 mmol, 1 eq) in MeCN/H2O (4: 1, 130 mL) between -5 ℃ to -10 ℃ and stirred for 2 hs . After the reaction was complete, Na2S2O3 (10%, 20 ml) was added and stirred for 0.5 h at rt then separated . The water phase was re-extracted by DCM (50 mL *2) , the combine organic phase was concentrated, dissolved by MTBE (1V) , the solid was filtered off to recover the auxiliary, the filtrate was concentrated to dryness to used in the next step.
[0254]
Alternative Method 1b: N-chlorosuccinimide (13.3 g, 1.1 eq) was added portionwisely to a solution of (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (12) (25.9 g, 100 mmol, 1 eq) in 100ml CH3CN at -5 ℃, and stirred for 2 hs . After the reaction was complete, NaHCO3 (sat, 20 mL) was added and stirred for 0.5 h at rt. The mixture were extracted by DCM (50 mL *2) , washed with brine and dried over MgSO4. Solvents were removed, the residue dissolved by MTBE (1V) , the solid was filtered off to recover the auxiliary, the filtrate was concentrated to dryness to obtained the (3R, 4R, 5R) -5- (chloromethyl) -3-fluoro-4-hydroxy-3-methyldihydrofuran-2 (3H) -one (18b) , m/z (ES+) : 183 [M+H] +.
[0255]
The related lactone 18a or 18b (0.14eq) was dissolved in EtOH (104 mL) , then KOH (30%in H2O, 50 mL) was added into, the result mixture was reflux for 4 hs. Then HCl (16.7 mL, 12 M) was added into the mixture and reflux for another 2 hs. The solvent was removed and the residue was recrystalized in toluene to give the desired compound as a white solid (yield: 80~85%) . m/z (ES+) : 165 [M+H] +. 1H-NMR (400 MHz, MeOD) : δ 4.34 (ddd, J = 8.0, 4.2, 2.3 Hz, 1 H) , 4.02 (ddd, J = 17.6, 15.2, 5.1 Hz, 2 H) , 3.74 (dd, J = 13.0, 4.2 Hz, 1 H) , 1.60 (s, 1.5 H) , 1.54 (s, 1.5 H) ; 19F-NMR (400 MHz, MeOD) : -172.47 ppm.
[0258]
Osmium tetroxide (OsO4) (0.1 equiv) was added in one portion to a stirring solution of the (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyloxazolidin-2-one (12) (25.9 g, 100 mmol, 1 eq) in acetone/water (8: 1 ratio) under nitrogen. After 5 min, NMO (N-methylmorpholine N-oxide, 60%by weight in water, 1.1 equiv) was added in one portion and stirred for 24 h. The resulting reaction mixture was concentrated under reduced pressure and immediately purified via column chromatography to obtain the desired lactone (3R, 4R, 5S) -3-fluoro-4-hydroxy-5- (hydroxymethyl) -3-methyldihydrofuran-2 (3H) -one (21) , yield: 87%, m/z (ES+) : 165 [M+H] +.
[0259]
15.1 g (92.3 mmol) (3R, 4R, 5S) -3-fluoro-4-hydroxy-5- (hydroxymethyl) -3-methyl dihydrofuran-2 (3H) -one (21) was dissolved in 25 mL pyridine and 11.1 g (96.9 mmol) methanesulfonyl chloride was slowly added dropwise at -25 degC. It was stirred for a day at -25 deg and a day at -10 deg. After adding 20 mL of ethyl acetate and 20 mL water, the solvent was removed on a rotary evaporator. After neutralization with dilute sodium hydrogen carbonate solution, the solvent was removed in vacuo again, the residue was digested with ethyl acetate, the eluate was dried with magnesium sulfate and concentrated in vacuo to dryness. Recrystallization from ethyl acetate/diethyl ether gave a colorless crystalline product ( (2S, 3R, 4R) -4-fluoro-3-hydroxy-4-methyl-5-oxotetrahydrofuran-2-yl) methyl methanesulfonate (18c) . Yield: 31 %.
[0260]
33.8g of ( (2S, 3R, 4R) -4-fluoro-3-hydroxy-4-methyl-5-oxotetrahydrofuran-2-yl) methyl methanesulfonate was disslolved in EtOH (104 mL) , then KOH (16.8 g , 3 eq) in H2O (52 mL) was added into, the result mixture was reflux for 4 hs. Then HCl (16.7 mL, 12 M) was added into, the mixture was reflux for another 2 hs. The solvent was removed and the residue was recrystalized in toluene to give the desired compound as a white solid (10.5 g, yield: 45%) .
[0261]
Alternative reagents and reactions to those disclosed above can also be employed. For example, 4-methylbenzene-1-sulfonyl chloride can be used instead of methanesulfonyl chloride. Moreover, primary alcohol can be converted to chloro or bromo by using Ph3P/CCl4, PPh3P/CBr4, PPh3/NCS, PPh3/NBS, or PPh3/C2Cl6 as a halogenation reagent. The desired product can be obtained in good yields using these reagents and reactions.
[0262]
Method 3: Using a method analogous to that described as hereinabove and (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methyl pent-4-enoyl) -4-isopropyloxazolidin-2-one (13) as starting material provides the desired compound 19 (yield: 63.2%)
[0263]
Method 4: Using a method analogous to that described as hereinabove and (S) -4-benzyl-3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) oxazolidin-2-one (14) as starting material provides the desired compound 19 (yield: 71.8%)
[0264]
Method 5: Using a method analogous to that described as hereinabove and (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-phenyloxazolidin-2-one (15) as the start material gives the desired compound 19 (yield: 65.7%)
[0265]
Method 6: Using a method analogous to that described as hereinabove and (R) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-phenyloxazolidin-2-oneas (16) starting material provides the desired compound 19 (yield: 59.5%)
[0266]
Method 7: Using a method analogous to that described as hereinabove and (S) -3- ( (2R, 3R) -2-fluoro-3-hydroxy-2-methylpent-4-enoyl) -4-isopropyl-5, 5-diphenyloxazolidin-2-one (17) as starting material gives the desired compound 19 (yield: 66.7%)
[0267]
Example 17: preparation of ( (3R, 4R) -3- (benzoyloxy) -4-fluoro-4-methyl-5-oxotetra hydro fur an-2-yl) methyl benzoate
[0269]
(3R, 4R) -3-fluoro-4-hydroxy-5- (hydroxymethyl) -3-methyldihydrofuran-2 (3H) -one (19) (25.4 g, 0.154 mol) obtained from example 3 was dissolved in 200 ml of THF. 4- (Dimethylamino) -pyridine (8.2 g, 0.066 mol) and triethylamine (35 g, 0.35 mol) were added and the reaction mixture was cooled to 0 ℃. Benzoyl chloride (46.0 g, 0.33 mol) was added, and the mixture was warmed to 35-40 ℃ in the course of 2 hs. Upon completion of the reaction, water (100 mL) was charged and the mixture was stirred for 30 min. Phases were separated and to the aqueous phase methyl-tert-butyl ether (100 mL) was added and the mixture was stirred for 30 min. Phases were separated and the organic phase was washed with saturated NaCl solution (100 mL) . The combined organic phases were dried over Na2SO4 (20 g) filtered and the filtrate was evaporated to dryness. The residue was taken up in iso-propanol (250 mL) and the mixture was warmed to 50 ℃ and stirred for 60 min, then cooled down to 0 ℃ and further stirred for 60 min. The solid was filtered and the wet cake was washed with i-propanol (50 mL) and then dried under vacuum. The title compound ( (3R, 4R) -3- (benzoyloxy) -4-fluoro-4-methyl-5-oxotetrahydrofuran-2-yl) methyl benzoate (47.5 g, 82.6 %yield) was obtained. ‘H-NMR (CDCl3, 400 MHz) : 8.10 (d, 7=7.6 Hz, 2H) , 8.00 (d, 7=7.6 Hz, 2H) , 7.66 (t, 7=7.6 Hz, IH) , 7.59 (t, 7=7.6 Hz, IH) , 7.50 (m, 2H) , 7.43 (m, 2H) , 5.53 (dd, 7=17.6, 5.6 Hz, IH) , 5.02 (m, IH) , 4.77 (dd, 7=12.8, 3.6 Hz, IH) , 4.62 (dd, 7=12.8, 5.2 Hz, IH) , 1.77 (d, 7=23.2 Hz, 3H) .













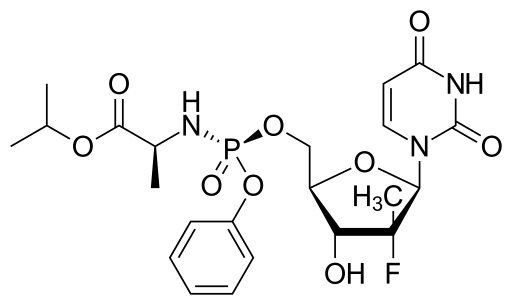 SOFOSBUVIR
SOFOSBUVIR

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..

 LIONEL MY SON
LIONEL MY SON

























