An efficient preparation of a precursor to the neprilysin inhibitor sacubitril is described. The convergent synthesis features a diastereoselective Reformatsky-type carbethoxyallylation and a rhodium-catalyzed stereoselective hydrogenation for installation of the two key stereocenters. Moreover, by integrating machine-assisted methods with batch processes, this procedure allows a safe and rapid production of the key intermediates which are promptly transformed to the target molecule (3·HCl) over 7 steps in 54% overall yield.
Synthesis of a Precursor to Sacubitril Using Enabling Technologies
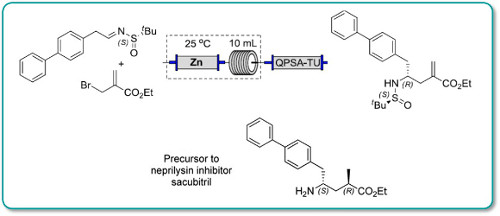
Continuous flow methodologyhas been used to enhance several steps in the synthesis of a precursor to Sacubitril.
In particular, a key carboethoxyallylation benefited from a reducedprocessing time and improved reproducibility, the latter attributable toavoiding the use of a slurry as in the batch procedure. Moreover, in batchexothermic formation of the organozinc species resulted in the formation ofside products, whereas this could be avoided in flow because heat dissipationfrom a narrow packed column of zinc was more efficient
Synthesis of a Precursor to Sacubitril Using Enabling Technologies

LCZ696 (sacubitril/valsartan) is a first-in-class combination of the angiotensin II receptor-blocker valsartan and the neprilysin inhibitor sacubitril. A recent head-to-head comparison of LCZ696 with enalapril in a double-blind trial was stopped early because the boundary for an overwhelming benefit with LCZ696 was crossed.As a result of this, LCZ696 was reviewed under the FDA’s priority review program and was granted approval on the July 7, 2015 to reduce the risk of cardiovascular death and hospitalization for HF in patients with chronic HF (NYHA Class II–IV) and reduced ejection fraction.
LCZ696 is a complex aggregate comprised of the anionic forms of sacubitril and valsartan, sodium cations, and water molecules in the molar ratio of 1:1:3:2.5, respectively

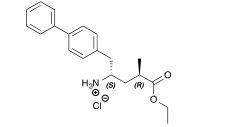
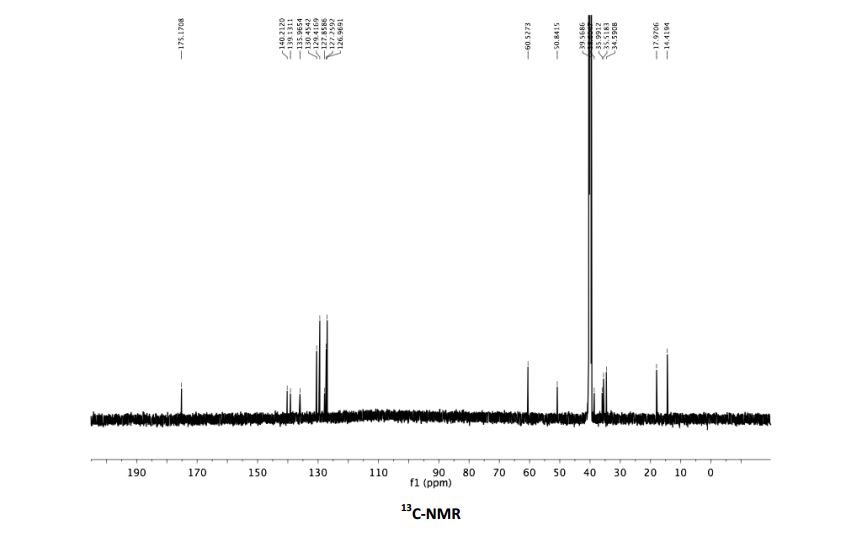
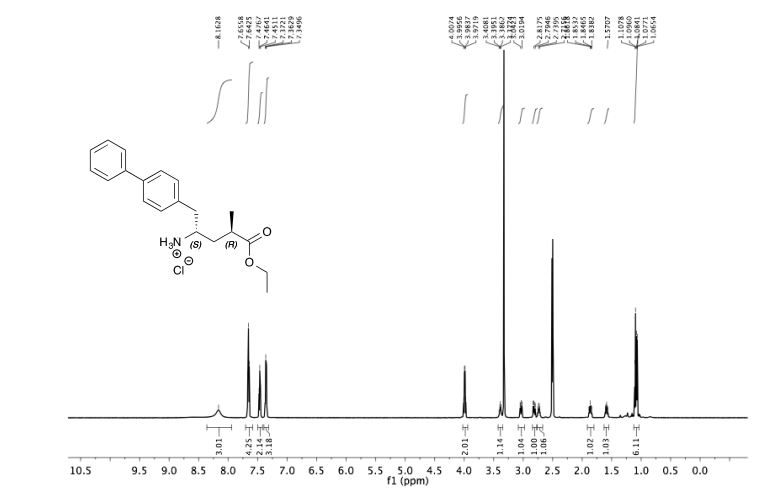
(2R, 4S)-5-(4-biphenylyl)-4-amino-2-methylpentanoic acid ethyl ester hydrochloride 3
To a stirred solution of (2R, 4S)-5-(4-Biphenylyl)-2-methyl-4-(tert-butylsulfinylamino)valeric acid 14 (50.0 mg, 134 μmol) in absolute ethanol (0.4 mL) at 0 °C was added thionyl chloride (20 μL, 268 μmol). The reaction mixture was stirred at room temperature for 3 h. The solvent was removed to yield 46.0 mg (99%) of titled compound 3 as a white solid.
1 H NMR (600 MHz, DMSO-d6) δ 8.17 (br. s, 3H), 7.66 (dd, J = 8.0, 7.4 Hz, 4H), 7.47 (t, J = 7.7 Hz, 2H), 7.36 (2 H, t, J = 7.4 Hz, H15, 2H), 7.36 (1 H, d, J = 8.0 Hz, H15), 3.99 (q, J = 7.1 Hz, H18), 3.42 – 3.36 (m, H4, 1H), 3.04 (dd, J = 13.8, 5.5 Hz, 1H), 2.81 (dd, J = 13.8, 8.1 Hz, 1H), 2.77 – 2.70 (m, 1H), 1.86 (ddd, J = 14.3, 9.1, 5.0 Hz, 1H), 1.59 (ddd, J = 13.8, 8.1, 5.4 Hz, 1H), 1.10 (t, J = 7.1 Hz, 3H), 1.07 (d, J = 7.1 Hz, 3H).
13C NMR (151 MHz, CDCl3) δ 174.7, 139.7, 138.7, 135.5, 130.0, 129.0, 127.4, 126.8, 126.5, 60.1, 50.4, 38.1, 35.5, 35.0, 17.5, 13.9.
HRMS (ESI+ , m/z [M+H]+ ) Calcd for C20H26NO2 312.1964; found 312.1967;
HPLC. 97:3 d.r. (Daicel Chiralpak AD-H column; isocratic n-hexane/ethanol/methanol/trimethylamine 80/10/10/0.2; 40 o C; flow rate = 0.8 mL min-1 ; λ = 254 nm; run time = 23 mins; tR (2R, 4S) 97.07%; tR (2S,4R) 0.21%; tR (2S, 4S) 2.32%; tR (2R,4R) 0.40%)
13C NMR Ethyl (2R,4S)-5-(4-biphenylyl)-4-amino-2-methylpentanoate hydrochloride 3
////////////Synthesis, Precursor, Sacubitril, Enabling Technologies, flow synthesis, valsartan, LCZ69