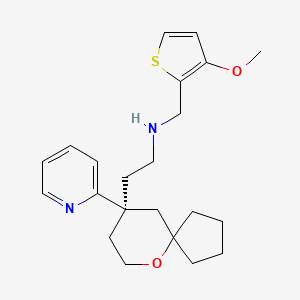
Oliceridine
N-[(3-methoxythiophen-2-yl)methyl]-2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-1-amine
[(3-Methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9- yl]ethyl})amine
Phase III
A mu-opioid receptor ligand potentially for treatment of acute postoperative pain.
![]()
TRV-130; TRV-130A
CAS No.1401028-24-7
| Molecular Formula: | C22H30N2O2S |
|---|---|
| Molecular Weight: | 386.5508 g/mol |
- Originator Trevena

- Class Analgesics; Small molecules
- Mechanism of Action Beta arrestin inhibitors; Opioid mu receptor agonists
- Orphan Drug Status No
- On Fast track Postoperative pain
-
- Phase III Postoperative pain
- Phase II Pain
Most Recent Events
- 09 Mar 2016Trevena intends to submit NDA to US FDA in 2017
- 22 Feb 2016Oliceridine receives Breakthrough Therapy status for Pain in USA
- 19 Jan 2016Phase-III clinical trials in Postoperative pain in USA (IV) (NCT02656875)
Oliceridine (TRV130) is an opioid drug that is under evaluation in human clinical trials for the treatment of acute severe pain. It is afunctionally selective μ-opioid receptor agonist developed by Trevena Inc. Oliceridine elicits robust G protein signaling, with potencyand efficacy similar to morphine, but with far less β-arrestin 2 recruitment and receptor internalization, it displays less adverse effectsthan morphine.[1][2][3]
In 2015, the product was granted fast track designation in the U.S. for the treatment of moderate to severe acute pain. In 2016, the compound was granted FDA breakthrough therapy designation for the management of moderate to severe acute pain.
Oliceridine (TRV130) is an intravenous G protein biased ligand that targets the mu opioid receptor. Trevena is developing TRV130 for the treatment of moderate to severe acute pain where intravenous therapy is preferred, with a clinical development focus in acute postoperative pain
TRV 130 HCl is a novel μ-opioid receptor (MOR) G protein-biased ligand; elicits robust G protein signaling(pEC50=8.1), with potency and efficacy similar to morphine, but with far less beta-arrestin recruitment and receptor internalization.
NMR
Oliceridine (TRV130) – Mu Opioid Biased Ligand for Acute Pain
| Target | Indication | Ownership | ||
| Oliceridine (TRV130) | Mu-receptor | Moderate to Severe Pain |
 |
Oliceridine (TRV130) is an intravenous G protein biased ligand that targets the mu opioid receptor. Trevena is developing TRV130 for the treatment of moderate to severe acute pain where intravenous therapy is preferred, with a clinical development focus in acute postoperative pain.
Recent TRV130 News
- 05/02/16 – Trevena Announces Successful End-of-Phase 2 Meeting with FDA and Outlines Phase 3 Program for Oliceridine
- 03/31/16 – Trevena Announces Presentations at the 41st Annual Regional Anesthesiology and Acute Pain Medicine Meeting
- 02/22/16 – Trevena, Inc. Receives FDA Breakthrough Therapy Designation for Oliceridine
Opioid receptors (ORs) mediate the actions of morphine and morphine-like opioids, including most clinical analgesics. Three molecularly and pharmacologically distinct opioid receptor types have been described: δ, κ and μ. Furthermore, each type is believed to have sub-types. All three of these opioid receptor types appear to share the same functional mechanisms at a cellular level. For example, activation of the opioid receptors causes inhibition of adenylate cyclase, and recruits β-arrestin.
When therapeutic doses of morphine are given to patients with pain, the patients report that the pain is less intense, less discomforting, or entirely gone. In addition to experiencing relief of distress, some patients experience euphoria. However, when morphine in a selected pain-relieving dose is given to a pain-free individual, the experience is not always pleasant; nausea is common, and vomiting may also occur. Drowsiness, inability to concentrate, difficulty in mentation, apathy, lessened physical activity, reduced visual acuity, and lethargy may ensue.
There is a continuing need for new OR modulators to be used as analgesics. There is a further need for OR agonists as analgesics having reduced side effects. There is a further need for OR agonists as analgesics having reduced side effects for the treatment of pain, immune dysfunction, inflammation, esophageal reflux, neurological and psychiatric conditions, urological and reproductive conditions, medicaments for drug and alcohol abuse, agents for treating gastritis and diarrhea, cardiovascular agents and/or agents for the treatment of respiratory diseases and cough.
PAPER
Structure activity relationships and discovery of a g protein biased mu opioid receptor ligand, ((3-Methoxythiophen-2-yl)methyl)a2((9R)-9-(pyridin-2-y1)-6-oxaspiro-(4.5)clecan-9-yl)ethylpamine (TRV130), for the treatment of acute severe pain
J Med Chem 2013, 56(20): 8019
Structure–Activity Relationships and Discovery of a G Protein Biased μ Opioid Receptor Ligand, [(3-Methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the Treatment of Acute Severe Pain
Abstract

The concept of “ligand bias” at G protein coupled receptors has been introduced to describe ligands which preferentially stimulate one intracellular signaling pathway over another. There is growing interest in developing biased G protein coupled receptor ligands to yield safer, better tolerated, and more efficacious drugs. The classical μ opioid morphine elicited increased efficacy and duration of analgesic response with reduced side effects in β-arrestin-2 knockout mice compared to wild-type mice, suggesting that G protein biased μ opioid receptor agonists would be more efficacious with reduced adverse events. Here we describe our efforts to identify a potent, selective, and G protein biased μ opioid receptor agonist, TRV130 ((R)-30). This novel molecule demonstrated an improved therapeutic index (analgesia vs adverse effects) in rodent models and characteristics appropriate for clinical development. It is currently being evaluated in human clinical trials for the treatment of acute severe pain.
[(3-Methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl] ethyl})amine ((R)-30)
Patent
WO 2012129495
http://www.google.com/patents/WO2012129495A1?cl=en
Scheme 1: Synthesis of Spirocyclic Nitrile
NCCH2C02CH3 AcOH, NH4OAc
1-5 1-6 1-7
Chiral HPLC separation n=1-2
R= phenyl, substituted phenyl, aryl,
s
Scheme 2: Converting the nitrile to the opioid receptor ligand (Approach 1)
2-4
Scheme 3: Converting the nitrile to the opioid receptor ligand (Approach 2)
1-8B 3-1 3-2 n=1-2
In some embodiments, the same scheme is applied to 1 -7 and 1 -8A. Scheme 4: Synthesis of Non-Spirocyclic Nitrile
4-1 4-2 4-3
KOH, ethylene glycol R= phenyl, substituted phenyl, aryl,
substituted aryl, pyridyl, substituted pyridyl, heat heteroaryl, substituted heteroaryl,
carbocycle, heterocycle and etc.
In some embodiments, 4-1 is selected from the group consisting of
4-1 A 4-1 B 4-1 C 4-1 D 4-1 E
Scheme 5: Synthesis of Other Spirocyclic Derived Opioid Ligands
5-1 5-2 5-3
Scheme 6: Allyltrimethylsilane Approach to Access the Quaternary Carbon Center
RMgX, or RLi
Scheme 7: N-linked Pyrrazole Opioid Receptor Ligand
[(3-Methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9- yl]ethyl})amine
Into a vial were added 2-[(9R)-9-(Pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-l -amine (500 mg, 1.92 mmole), 18 mL CH2C12 and sodium sulfate (1.3 g, 9.6 mmole). The 3- methoxythiophene-2-carboxaldehyde (354 mg, 2.4 mmole) was then added, and the misture was stirred overnight. NaBH4 (94 mg, 2.4 mmole) was added to the reaction mixture, stirred for 10 minutes, and then MeOH (6.0 mL) was added, stirred l h, and finally quenched with water. The organics were separated off and evaporated. The crude residue was purified by a Gilson prep HPLC. The desired fractions collected and concentrated and lyophilized. After lyophilization, residue was partitioned between CH2C12 and 2N NaOH, and the organic layers were collected. After solvent was concentrated to half of the volume, 1.0 eq of IN HC1 in Et20 was added,and majority of solvent evaporated under reduced pressure. The solid obtained was washed several times with Et20 and dried to provide [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2- yl)-6-oxaspiro[4.5]decan-9-yl]ethyl})amine monohydrochloride (336 mg, 41% yield, m/z 387.0 [M + H]+ observed) as a white solid. The NMR for Compound 140 is described herein.
Example 15: Synthesis of [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9- (pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethyl})amine (Compound 140).
Methyl 2-cyano-2-[6-oxaspiro[4.5]decan-9-ylidene]acetate (mixture of E and Z isomers)
A mixture of 6-oxaspiro[4.5]decan-9-one (13.74 g, 89.1 mmol), methylcyanoacetate (9.4 ml, 106.9 mmol), ammonium acetate (1.79 g, 26.17.mmol) and acetic acid (1.02 ml, 17.8 mmol) in benzene (75 ml) was heated at reflux in a 250 ml round bottom flask equipped with a Dean-Stark and a reflux condenser. After 3h, TLC (25%EtOAc in hexane, PMA stain) showed the reaction was completed. After cooling, benzene (50 ml) was added and the layer was separated, the organic was washed by water (120 ml) and the aqueous layer was extracted by CH2CI2 (3 x 120 ml). The combined organic was washed with sat’d NaHCCb, brine, dried and concentrated and the residual was purified by flash chromatography (340 g silica gel column, eluted by EtOAc in hexane: 5% EtOAc, 2CV; 5-25%, 14CV; 25-40%,8 CV) gave a mixture of E and Z isomers: methyl 2-cyano-2-[6- oxaspiro[4.5]decan-9-ylidene]acetate ( 18.37 g, 87.8 % yield, m/z 236.0 [M + H]+ observed) as a clear oil. -cyano-2-[9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]acetate
A solution of 2-bromopyridine (14.4 ml, 150 mmo) in THF (75 ml) was added dropwise to a solution of isopropylmagnesium chloride (75 ml, 2M in THF) at 0°C under N2, the mixture was then stirred at rt for 3h, copper Iodide(2.59 g, 13.6 mmol) was added and allowed to stir at rt for another 30 min before a solution of a mixture of E and Z isomers of methyl 2-cyano-2-[6-oxaspiro[4.5]decan-9-ylidene]acetate (16 g, 150 mmol) in THF (60 ml) was added in 30 min. The mixture was then stirred at rt for 18h. The reaction mixture was poured into a 200 g ice/2 N HC1 (100 ml) mixture. The product was extracted with Et20 (3×300 ml), washed with brine (200 ml), dried (Na2S04) and concentrated. The residual was purified by flash chromatography (100 g silica gel column, eluted by EtOAc in hexane: 3% 2CV; 3-25%, 12 CV; 25-40% 6CV gave methyl 2-cyano-2-[9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]acetate (15.44 g, 72% yield, m/z 315.0 [M + H]+ observed) as an amber oil .
-[9-(Pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]acetonitrile
Ethylene glycol (300 ml) was added to methyl 2-cyano-2-[9-(pyridin-2-yl)-6- oxaspiro[4.5]decan-9-yl]acetate( 15.43 g, 49 mmol) followed by potassium hydroxide (5.5 g , 98 mmol), the resulting mix was heated to 120oC, after 3 h, the reaction mix was cooled and water (300 ml) was added, the product was extracted by Et20(3 x 400 ml), washed with water(200 ml), dried (Na2S04) and concentrated, the residual was purified by flash chromatography (340 g silica gel column, eluted by EtOAc in hexane: 3% 2CV; 3-25%, 12 CV; 25-40% 6CV to give 2-[9-(Pyridin-2-yl)-6-oxaspiro[4.5]decan-9- yl]acetonitrile (10.37 g, 82% yield, m/z 257.0 [M + H]+ observed).
-yl)-6-oxaspiro[4.5]decan-9-yl]acetonitrile
racemic 2-[9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]acetonitrile was separated by chiral HPLC column under the following preparative-SFC conditions: Instrument: SFC-80 (Thar, Waters); Column: Chiralpak AD-H (Daicel); column temperature: 40 °C; Mobile phase: Methanol /CO2=40/60; Flow: 70 g/min; Back pressure: 120 Bar; Cycle time of stack injection: 6.0min; Load per injection: 225 mg; Under these conditions, 2-[9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]acetonitrile (4.0 g) was separated to provide the desired isomer, 2-[(9R)-9-(Pyridin-2-yI)-6- oxaspiro[4.5]decan-9-yl]acetonitrile (2.0 g, >99.5% enantiomeric excess) as a slow- moving fraction. The absolute (R) configuration of the desired isomer was later determined by an X-ray crystal structure analysis of Compound 140. [0240] -[(9R)-9-(Pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-l-amine
LAH (1M in Et20, 20ml, 20 mmol) was added to a solution of 2-[(9R)-9-(pyridin-2-yl)- 6-oxaspiro[4.5]decan-9-yl]acetonitrile (2.56 g, 10 mmol) in Et20 (100 ml, 0.1M ) at OoC under N2. The resulting mix was stirred and allowed to warm to room temperature. After 2 h, LCMS showed the reaction had completed. The reaction was cooled at OoC and quenched with water ( 1.12 ml), NaOH (10%, 2.24 ml) and another 3.36 ml of water. Solid was filtered and filter pad was washed with ether (3 x 20 ml). The combined organic was dried and concentrated to give 2-[(9R)-9-(Pyridin-2-yl)-6- oxaspiro[4.5]decan-9-yl]ethan-l -amine (2.44 g, 94% yield, m/z 260.6 [M + H]+ observed) as a light amber oil.
Alternatively, 2-[(9R)-9-(Pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-l -amine was prepared by Raney-Nickel catalyzed hydrogenation.
An autoclave vessel was charged with 2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4,5]decan-9- yl] acetonitrile and ammonia (7N solution in methanol). The resulting solution was stirred at ambient conditions for 15 minutes and treated with Raney 2800 Nickel, slurried in water. The vessel was pressurized to 30 psi with nitrogen and agitated briefly. The autoclave was vented and the nitrogen purge repeated additional two times. The vessel was pressurized to 30 psi with hydrogen and agitated briefly. The vessel was vented and purged with hydrogen two additional times. The vessel was pressurized to 85-90 psi with hydrogen and the mixture was warmed to 25-35 °C. The internal temperature was increased to 45-50 °C over 30-60 minutes. The reaction mixture was stirred at 45-50 °C for 3 days. The reaction was monitored by HPLC. Once reaction was deemed complete, it was cooled to ambient temperature and filtered through celite. The filter cake was washed with methanol (2 x). The combined filtrates were concentrated under reduced pressure at 40-45 °C. The resulting residue was co-evaporated with EtOH (3 x) and dried to a thick syrupy of 2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethan-l -amine.
References
- Chen XT, Pitis P, Liu G, Yuan C, Gotchev D, Cowan CL, Rominger DH, Koblish M, Dewire SM, Crombie AL, Violin JD, Yamashita DS (October 2013). “Structure-Activity Relationships and Discovery of a G Protein Biased μ Opioid Receptor Ligand, [(3-Methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the Treatment of Acute Severe Pain”. J. Med. Chem. 56 (20): 8019–31.doi:10.1021/jm4010829. PMID 24063433.
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD (March 2013). “A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine”. J. Pharmacol. Exp. Ther. 344 (3): 708–17.doi:10.1124/jpet.112.201616. PMID 23300227.
- Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan C, Violin JD, Lark MW (October 2013). “First clinical experience with TRV130: Pharmacokinetics and pharmacodynamics in healthy volunteers”. J Clin Pharmacol 54(3): 351–7. doi:10.1002/jcph.207. PMID 24122908.
External links
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015246904 | 2015-09-03 | Opioid Receptor Ligands And Methods Of Using And Making Same |
| US8835488 | 2014-09-16 | Opioid receptor ligands and methods of using and making same |
| US2013331408 | 2013-12-12 | Opioid Receptor Ligands and Methods of Using and Making Same |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
N-[(3-methoxythiophen-2-yl)methyl]-2-[(9R)-9-pyridin-2-yl-6-oxaspiro[4.5]decan-9-yl]ethanamine
|
|
| Clinical data | |
| Routes of administration |
IV |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | 1401028-24-7 |
| ATC code | none |
| PubChem | CID 66553195 |
| ChemSpider | 30841043 |
| UNII | MCN858TCP0 |
| ChEMBL | CHEMBL2443262 |
| Synonyms | TRV130 |
| Chemical data | |
| Formula | C22H30N2O2S |
| Molar mass | 386.55 g·mol−1 |
////////TRV-130; TRV-130A, Oliceridine, Phase III, Postoperative pain, trevena, mu-opioid receptor ligand, fast track designation, breakthrough therapy designation
COc1ccsc1CNCC[C@]2(CCOC3(CCCC3)C2)c4ccccn4