
FDA lifts clinical hold on Cangene’s hemophilia compound IB1001
WINNIPEG, July 29, 2013 /CNW/ – Cangene Corporation (Cangene) today announces that the U.S. Food and Drug Administration (FDA) has lifted the clinical hold previously placed on clinical trials evaluating the safety and efficacy of IB1001, a recombinant Factor IX (rFIX) product being developed for the treatment and prevention of bleeding episodes with hemophilia B.
http://www.pharmalive.com/fda-lifts-clinical-hold-on-cangenes-hemophilia-compoun
IB1001 is an intravenous recombinant Factor IX (rFIX) being developed for the treatment and prevention of bleeding episodes in people with hemophilia B. The IB1001 development program includes a comprehensive set of pharmacokinetic, safety, and efficacy data from a Phase 3 clinical trial in people affected by hemophilia B, including a surgery sub-study.
A biologics license application (BLA) was submitted to the U.S. Food and Drug Administration (FDA) in March 2012 and a Marketing Authorization Application (MAA) was submitted to the European Medicines Agency (EMA) in October 2011.
In July 2012, IB1001 was put on a clinical hold by the FDA due to a higher than expected rate of host cell antibody development in people treated with IB1001.
Cangene Corporation (TSX: CNJ) has completed its previously announced acquisition of the worldwide rights to investigational hemophilia compound IB1001 (recombinant FIX), as well as Inspiration’s rights to two product candidates in pre-clinical development: IB1007 (recombinant FVIIa) and IB1008 (recombinant FVIII) from Ipsen (Euronext: IPN; ADR: IPSEY) and Inspiration Biopharmaceuticals, Inc., in connection with Inspiration’s bankruptcy proceedings.
The transaction was slated to close on February 15, 2013.
Pursuant to the terms of the agreement, Cangene will pay approximately US $5.9 million upfront and up to $50 million in potential additional commercial milestones as well net sales payments equivalent to tiered double-digit percentage of IB1001 annual net sales.

-
amcrasto@gmail.com






















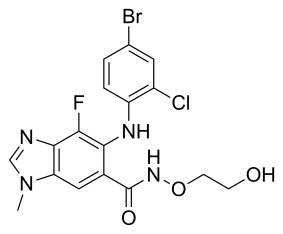



 LX4211
LX4211