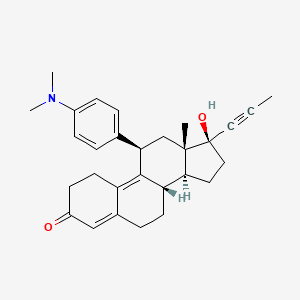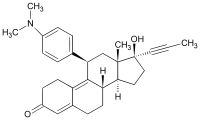
Mifepristone (or RU-486) is a synthetic steroid compound with both antiprogesterone and antiglucocorticoid properties. The compound is a 19-nor steroid with substitutions at positions C11 and C17 (17 beta-hydroxy-11 beta-[4-dimethylamino phenyl] 17 alpha-[1-propynyl]estra-4,9-dien-3-one), which antagonizes cortisol action competitively at the receptor level.
U.S. Pat. No. 4,386,085 (the ‘085 patent) discloses mifepristone starting from estra-5(10), 9(11)-diene-3,17-dione 3-ethylene acetal. The ‘085 patent discloses the purification of mifepristone by column chromatography using cyclohexane-ethyl acetate (7:3) mixture as an eluent. However, a drawback to the use of column chromatography is its unsuitability for industrial use.
Mifepristone is a progesterone receptor antagonist used as an abortifacient in the first months of pregnancy, and in smaller doses as an emergency contraceptive. Mifepristone is also a powerful glucocorticoid receptor antagonist, and has occasionally been used in refractory Cushing’s Syndrome(due to ectopic/neoplastic ACTH/Cortisol secretion). During early trials, it was known as RU-38486 or simply RU-486, its designation at the Roussel Uclaf company, which designed the drug. The drug was initially made available in France, and other countries then followed—often amid controversy. It is marketed under tradenames Korlym and Mifeprex, according to FDA Orange Book.
Mifepristone was the first antiprogestin to be developed and it has been evaluated extensively for its use as an abortifacient. The original target for the research group, however, was the discovery and development of compounds with antiglucocorticoid properties. It is these antiglucocorticoid properties that are of great interest in the treatment of severe mood disorders and psychosis.
In April 1980, as part of a formal research project at Roussel-Uclaf for the development of glucocorticoid receptorantagonists, chemist Georges Teutsch synthesized mifepristone (RU-38486, the 38,486th compound synthesized by Roussel-Uclaf from 1949 to 1980; shortened to RU-486); which was discovered to also be a progesterone receptor antagonist. In October 1981, endocrinologist Étienne-Émile Baulieu, a consultant to Roussel-Uclaf, arranged tests of its use for medical abortion in eleven women in Switzerland by gynecologist Walter Herrmann at theUniversity of Geneva‘s Cantonal Hospital, with successful results announced on April 19, 1982. On October 9, 1987, following worldwide clinical trials in 20,000 women of mifepristone with aprostaglandin analogue (initially sulprostone or gemeprost, later misoprostol) for medical abortion, Roussel-Uclaf sought approval in France for their use for medical abortion, with approval announced on September 23, 1988.
On October 21, 1988, in response to antiabortion protests and concerns of majority (54.5%) owner Hoechst AG of Germany, Roussel-Uclaf’s executives and board of directors voted 16 to 4 to stop distribution of mifepristone, which they announced on October 26, 1988. Two days later, the French government ordered Roussel-Uclaf to distribute mifepristone in the interests of public health.French Health Minister Claude Évin explained that: “I could not permit the abortion debate to deprive women of a product that represents medical progress. From the moment Government approval for the drug was granted, RU-486 became the moral property of women, not just the property of a drug company.” Following use by 34,000 women in France from April 1988 to February 1990 of mifepristone distributed free of charge, Roussel-Uclaf began selling Mifegyne (mifepristone) to hospitals in France in February 1990 at a price (negotiated with the French government) of $48 per 600 mg dose.
Mifegyne was subsequently approved in Great Britain on July 1, 1991, and in Sweden in September 1992, but until his retirement in late April 1994, Hoechst AG chairman Wolfgang Hilger, a devout Roman Catholic, blocked any further expansion in availability. On May 16, 1994, Roussel-Uclaf announced that it was donating without remuneration all rights for medical uses of mifepristone in the United States to the Population Council, which subsequently licensed mifepristone to Danco Laboratories, a new single-product company immune to antiabortion boycotts, which won FDA approval as Mifeprex on September 28, 2000.
On April 8, 1997, after buying the remaining 43.5% of Roussel-Uclaf stock in early 1997, Hoechst AG ($30 billion annual revenue) announced the end of its manufacture and sale of Mifegyne ($3.44 million annual revenue) and the transfer of all rights for medical uses of mifepristone outside of the United States to Exelgyn S.A., a new single-product company immune to antiabortion boycotts, whose CEO was former Roussel-Uclaf CEO Édouard Sakiz. In 1999, Exelgyn won approval of Mifegyne in 11 additional countries, and in 28 more countries over the following decade.
 mifepristone
mifepristone



The compound of structural formula 2 can be prepared from (+)-estrone in seven steps. Methylation of hydroxy group at C-3 in (+)-estrone, reduction of 17-ketone to 17β-alcohol followed by Birch reduction of ring A and mild hydrolysis of the enol ether to afford estra-17β-hydroxy-5(10)-en-3-one in four steps (Ref: Wilds, A. L. and Nelson, N. A. J. Am. Chem. Soc. 1953, 75, 5365-5369). This compound in another three steps, namely bromination and dehydrobrominatlon, ketalisation followed by Oppenauer oxidation yield compound having structural formula 2 (Ref: Perelman, M; Farkas, E.; Fornefield, E. J.; Kraay, R. J. and Rapala, B. T. J. Am. Chem. Soc. 1960, 82, 2402-2403).
U.S. Pat. No. 4,386,085 describes the synthesis of steroids of the general formula mentioned therein
- EXAMPLE 15 17β-hydroxy-11β-(4-dimethylaminophenyl)-17α (Propa-1 ,2-dienyl) estra-4 ,9-dien-3-one.Step A: 11β-(4-dimethylaminophenyl) 3,3 – / 1,2-ethane diyl bis (oxy) / 17α-(propa-1 ,2-dienyl) estr-9-en-5α-17β-diol and 11β – (4 – dimethylaminophenyl) 3,3 – / 1,2-ethane diyl bis (oxy) / 17α-(prop-2-ynyl) estr-9-en-5α (-17β-diol. Preparation of lithium compound.
- In 50 cm3 of anhydrous tetrahydrofuran at 0, +5 ° C, bubbled up Allène the absorption of 2.1 g. Cooled to -70 ° C. and 15 minutes in 23.9 cm3 of a 1.3 M solution of butyllithium in hexanne. The resulting mixture is stirred for 15 minutes at -70 ° C.
Condensation
- A solution of lithium derivative obtained above was added at -70 ° C in 25 minutes a solution of 3.5 g of the product obtained in Step A of Example 7 in 35 cm3 of anhydrous tetrahydrofuran. Stirred for 1 hour at -70 ° C, slowly poured into a saturated aqueous solution iced ammonium chloride. Extracted with ether, the organic phase washed with saturated sodium chloride, dried and the solvent evaporated. 3.4 g of product which was chromatographed on silica eluting with petroleum ether-ethyl acetate (1-1) to 1 mile triethylamine. Thus isolated: a) 1.73 g of isomer 17α-(propa-1 ,2-dienyl) F = 178 ° C. / Α / D = -32 ° ± 2 ° (c = 0.7% chloroform) b) 1.5 g of isomer 17o (- (prop-2-ynyl) F = 150 ° C. / α / D = -15 ° ± 2 ° (c = 0.9% chloroform).
Step B: 17β-hydroxy-11β-(4-dimethylaminophenyl)-17α (propa-1, 2 – dienyl) estra-4 ,9-dien-3-one.
- Inert gas mixing 1.73 g of 17α isomer (- (propa-1, 2 – dienyl) obtained in Step A, 51.8 cm3 of 95% ethanol and 3.5 cm3 of 2N hydrochloric acid. stirred at 20 ° C for 1 hour, add 50 cm3 of methylene chloride and 50 cm3 of a 0.25 M solution of sodium bicarbonate, decanted, extracted with methylene chloride, washed with water, dried and the solvent evaporated. obtained 1.51 g of product was dissolved in 10 cm3 of methylene chloride hot. was added 15 cm3 of isopropyl ether, concentrated and allowed to stand. thus isolated 1.23 g of the expected product was crystallized again in methylene chloride-isopropyl ether. finally obtained 1.11 g of the expected product. F = 228 ° C.
/ Α / D – 139, 5 ° ± 3 ° (c = 0.8% chloroform). ANY ERROr MAIL ME amcrasto@gmail.com
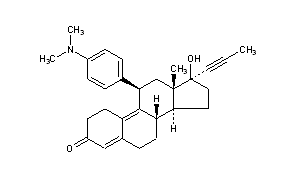 mifepristone
mifepristone| EP0817769 | 5-27-1999 | BORNEOL ESTERS, PROCESS FOR THEIR PREPARATION AND THEIR PHARMACEUTICAL USE |
| WO9907692 | 2-19-1999 | NEW EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING SAME AND THEIR PHARMACEUTICAL USE NEW EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING SAME AND THEIR PHARMACEUTICAL USE |
| EP0896819 | 2-18-1999 | Use of insect pheromones to treat pathologies induced by excess of glucocorticoid ANTIGLUCOCORTICOID DRUG |
| EP0758316 | 11-19-1998 | BORNEOL DERIVATIVES AFFECTING TUBULIN POLYMERIZATION AND DEPOLYMERIZATION |
| WO9735839 | 10-3-1997 | NOVEL BORNEOLS, PROCESSES FOR PRODUCING THEM AND PHARMACEUTICAL USE THEREOF |
| EP0648778 | 8-14-1997 | 11-Benzaldoximeestradiene-derivates, a process for their preparation and pharmaceutical compositions containing them |
| EP0648779 | 3-6-1997 | New 11-benzaldehyde oxime, 17-beta methoxy, 17 alpha methoxymethyl derivates of estradiene, a process for their preparation and pharmaceutical compositions containing them |
| US5450857 | 9-20-1995 | Method for the diagnosis of cervical changes |
| US5272140 | 12-22-1993 | 11-aryl steroid derivatives |
| WO0049019 | 8-25-2000 | NOVEL EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING THEM AND THEIR PHARMACEUTICAL USE NOVEL EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING THEM AND THEIR PHARMACEUTICAL USE |
| WO0049020 | 8-25-2000 | NOVEL EPOTHILON DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND THEIR PHARMACEUTICAL APPLICATION NOVEL EPOTHILON DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND THEIR PHARMACEUTICAL APPLICATION |
| WO0049021 | 8-25-2000 | 16-HALOGEN-EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING THEM AND THEIR PHARMACEUTICAL USE 16-HALOGEN-EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING THEM AND THEIR PHARMACEUTICAL USE |
| WO0047584 | 8-18-2000 | EPOTHILON DERIVATIVES, METHOD FOR THE PRODUCTION AND THE USE THEREOF AS PHARMACEUTICALS EPOTHILON DERIVATIVES, METHOD FOR THE PRODUCTION AND THE USE THEREOF AS PHARMACEUTICALS |
| WO0000485 | 1-7-2000 | EPOTHILON DERIVATIVES, THEIR PREPARATION PROCESS, INTERMEDIATE PRODUCTS AND THEIR PHARMACEUTICAL USE |
| WO9964393 | 12-17-1999 | NOVEL ANTIESTROGENS, A METHOD FOR THE PRODUCTION THEREOF, AND THEIR PHARMACEUTICAL APPLICATION |
| WO9959596 | 11-26-1999 | GLUCOCORTICOID RECEPTOR ANTAGONISTS FOR THE TREATMENT OF DEMENTIA |
| US5965623 | 10-13-1999 | Anti-glucocorticoid drug |
| EP0909764 | 9-30-1999 | 11 Beta-Benzaldoxim-9 Alpha, 10 Alpha-epoxy-estr-4-en-derivatives, a process for their production and pharmaceutical compositions containing them |
| WO9945023 | 9-11-1999 | S-SUBSTITUTED 11 beta -BENZALDOXIME-ESTRA-4,9-DIENE-CARBONIC ACID THIOLESTERS, METHOD FOR THE PRODUCTION THEREOF AND PHARMACEUTICAL PREPARATIONS CONTAINING THESE COMPOUNDS |
| EP0595133 | 1-6-2011 | PRODRUGS, THEIR PREPARATION AND USE AS MEDICAMENTS |
| US2010135956 | 6-4-2010 | STEROID MODULATORS OF PROGESTERONE RECEPTOR AND/OR GLUCOCORTICOID RECEPTOR |
| EP1005465 | 7-26-2007 | NEW EPOTHILONE DERIVATIVES, METHOD FOR PRODUCING SAME AND THEIR PHARMACEUTICAL USE |
| US2007105828 | 5-11-2007 | Novel polymorph form M of mifepristone and process for its preparation |
| EP0879605 | 7-27-2006 | GLYCOSYL PRODRUG CONJUGATE WITH ENHANCED TOLERANCE |
| EP1023074 | 7-13-2006 | METHODS FOR TREATING PSYCHOSIS ASSOCIATED WITH GLUCOCORTICOID RELATED DYSFUNCTION |
| EP0795334 | 2-2-2006 | Prodrugs for the treatment tumors and inflammatory diseases |
| EP0730448 | 2-7-2002 | USE OF NITRIC OXIDE SYNTHASE SUBSTRATE AND/OR DONOR, OR A NITRIC OXIDE INHIBITOR FOR THE MANUFACTURE OF MEDICAMENTS FOR THE TREATMENT OF UTERINE CONTRACTILITY DISORDERS |
| EP0809627 | 9-13-2001 | NOVEL BORNEOL DERIVATIVES, METHODS OF MANUFACTURING THEM, AND THEIR PHARMACEUTICAL USE |
| US6150349 | 11-22-2000 | Methods for treating psychosis associated with glucocorticoid related dysfunction |