Miconazole
416.13 [22916–47–8]
Miconazole Nitrate
C18H14Cl4N2O.HNO3 479.14 [22832–87–7]
MORE GRAPHS
13C
![]()
![]()
2D [1H,1H]-TOCSY BELOW
![]()
1D DEPT90
![]()
1D DEPT135
![]()
2D [1H,13C]-HSQC
![]()
2D [1H,13C]-HMBC
![]()
2D [1H,1H]-COSY
![]()
2D [1H,13C]-HMQC
![]()
Miconazole is an imidazole antifungal agent, developed by Janssen Pharmaceutica, commonly applied topically to the skin or tomucous membranes to cure fungal infections. It works by inhibiting the synthesis of ergosterol, a critical component of fungal cell membranes. It can also be used against certain species of Leishmania protozoa which are a type of unicellular parasites that also contain ergosterol in their cell membranes. In addition to its antifungal and antiparasitic actions, it also has some antibacterialproperties. It is marketed in various formulations under various brand names.
![]()

Brief background information
| Salt | ATC | Formula | MM | CAS |
|---|---|---|---|---|
| – | A01AB09 A07AC01 D01AC02 G01AF04 J02AB01 S02AA13 |
C 18 H 14 Cl 4 N 2 O | 416.14 g / mol | 22916-47-8 |
| mononitrate | A01AB09 A07AC01 D01AC02 G01AF04 J02AB01 S02AA13 |
C 18 H 14 Cl 4 N 2 O ⋅ HNO 3 | 479.15 g / mol | 22832-87-7 |
Using
-
antifungal agent for topical use
-
antimycotic agent
Classes substance
-
Imidazoles, 1- (hlorfenetil) imidazoles
synthesis Way
trade names
| A country | Tradename | Manufacturer |
|---|---|---|
| Germany | Castellani | Hollborn |
| Daktar | McNeil | |
| Derma-Mikotral | Rosen Pharma | |
| Fungur | HEXAL | |
| Gyno-Daktar | Janssen-Cilag, 1974 | |
| Gyno-Mikotral | Rosen Pharma | |
| Infektozoor Mundgel | Infectopharm | |
| Mikobeta | betapharm | |
| Mikotar | Dermapharm | |
| Mikoderm | Engelhard | |
| Mikotin | Ardeypharm | |
| Vobamik | Almirall Hermal | |
| France | Daktapin | Janssen-Cilag |
| Gyno-Daktapin | Janssen-Cilag | |
| Loramik | Bioalliance | |
| United Kingdom | Gyno-Daktapin | Janssen-Cilag |
| Italy | Daktapin | Janssen-Cilag |
| Mikonal | Ecobi | |
| Mikotef | LPB | |
| Miderm | Mendelejeff | |
| Nizakol | PS Pharma | |
| Pivanazolo | Medestea | |
| Prilagin | Sofar | |
| Japan | Florid | Mochida |
| USA | Fungoid | Pedinol |
| Ukraine | GІNEZOL 7 | Sagmel, Іnk., USA |
| MІKONAZOL-Darnitsa | CJSC “Farmatsevtichna FIRMA” Darnitsa “, m. Kyiv, Ukraine | |
| MІKOGEL | BAT “Kiїvmedpreparat”, m. Kyiv, Ukraine | |
| various generic drugs | ||
Formulations
-
ampoule 200 mg / 20 ml;
-
cream 1%, 2 g / 100 g 20 mg / g;
-
losyon 1%;
-
ointment 1%;
-
2% oral gel;
-
Powder 2 g / 100 g 20 mg / g (in the form mononitrate);
-
solution of 20 mg / ml;
-
100 mg suppositories;
-
Tablets of 250 mg (free base form);
-
vaginal cream 20 mg / g;
-
bottles of 400 mg / 40 ml
references
-
Synthesis of a)
-
DAS 1,940,388 (Janssen; appl 8.8.1969;. USA-prior 19.8.1968, 23.7.1969.).
-
US 3,717,655 (Janssen; 20.2.1973; appl 19.8.1968.).
-
US 3,839,574 (Janssen; 1.10.1974; prior 23.7.1969.).
-
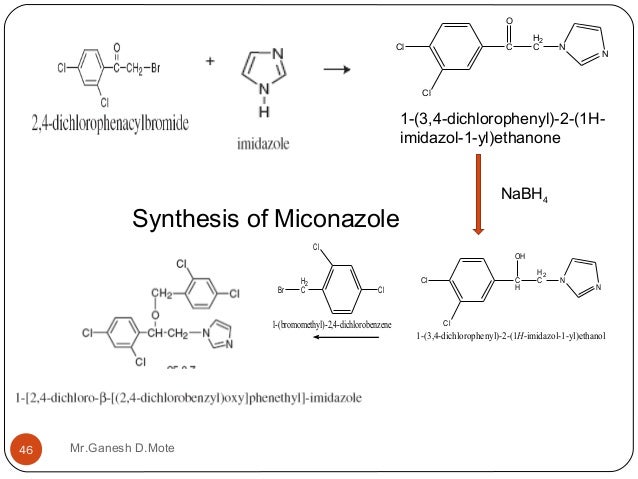
Miconazole nitrate was prepared by Godefori et
al [5–7]. Imidazole 1 was coupled with
brominated 2,4‑dichloroacetophenone 2 and the resulting ketonic product 3
was reduced with sodium borohydride to its corresponding alcohol 4. The
latter compound 4 was then coupled with 2,4-dichlorotoluene by sodium borohydride
in hexamethylphosphoramide (an aprotic solvent) which was then extracted with
nitric acid to give miconazole nitrate.
prepared by Molina Caprile [8] as follows:
1-phenyl-2-bromoethanone 2. Compound 2 was treated with
methylsulfonic acid to yield the corresponding methylsulfonate 3.
Etherification of 3 gave the a‑benzyloxy derivative 4 and compound 4 was
then chlorinated to give the 2,4‑dichlorinated derivative in both aromatic ring
systems 5. Compound 5 reacted with imidazole in dimethylformamide
to give miconazole 6 [7] which is converted to miconazole nitrate.
et al reported that the reduction of 2,4-dichlorophenyl-2-chloroethanone
1 with potassium borohydride in dimethylformamide to give 90% a‑chloromethyl-2,4-dichlorobenzyl
alcohol 2. Alkylation of imidazole with compound 2 in dimethylformamide
in the presence of sodium hydroxide and triethylbenzyl ammonium chloride, gave
1-(2,4‑dichlorophenyl-2-imidazolyl)ethanol 3 and etherification of 3
with 2,4-dichlorobenzyl chloride under the same condition, 62% yield of
miconazole [9].
and Li enantioselectively synthesized and studied the antifungal activity of
optically active miconazole and econazole. The key step was the
enantioselective reduction of 2‑chloro-1-(2,4-dichlorophenyl)ethanone catalyzed
by chiral oxazaborolidine [10].
et al reported the synthesiz of miconazole and analogs through a
carbenoid intermediate. The process involves the intermolecular insertion of
carbenoid species to imidazole from a‑diazoketones with copper acetylacetonate as the key
reaction of the synthetic route [11].
(1970).
E.F. Godefori and J. Heeres, U.S. Pat. 3,717,655
(1973).
E.F. Godefori, J. Heeres, J. van Cutsem and P.A.J.
Janssen, J. Med. Chem., 12, 784 (1969).
F. Molina Caprile, Spanish Patent ES 510870 A1
(1983).
B. Ye, K. Yu and Q. Huang, Zhongguo Yiyao Gongye
Zazhi, 21, 56 (1990).
Y.W. Liao and H.X. Li, Yaoxue Xuebao, 28,
22 (1993).
E.C. Yanez, A.C. Sanchez, J.M.S. Becerra, J.M.
Muchowski and C.R. Almanza, Revista de la Sociedad Quimica de Mexico, 48,
49 (2004).
![]() Title: Miconazole
Title: Miconazole
References
- ^ “WHO Model List of EssentialMedicines” (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ British National Formulary ’45’ March 2003
- ^ “Strange Beauty: Monistat Effectively Increases Hair Growth?”. Black Girl With Long Hair. Retrieved 12 April 2012.
- ^ Ju, Jiang; Tsuboi, Ryoji; Kojima, Yuko; Ogawa, Hideoki (2005). “Topical application of ketoconazole stimulates hair growth in C3H/HeN mice”. Journal of dermatology. 32: 243–247.
- ^ S., Venturoli; O. Marescalchi; F. M. Colombo; S. Macrelli; B. Ravaioli; A. Bagnoli; R. Paradisi; C. Flamigni (April 1999). “A Prospective Randomized Trial Comparing Low Dose Flutamide, Finasteride, Ketoconazole, and Cyproterone Acetate-Estrogen Regimens in the Treatment of Hirsutism”. The Journal of Clinical Endocrinology and Metabolism. 84 (4): 1304–1310. doi:10.1210/jc.84.4.1304. Retrieved 12 April 2012.
- ^ Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, Vilarem MJ, Maurel P, Gerbal-Chaloin S (2006). “Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor”. Mol. Pharmacol. 70 (1): 329–39. doi:10.1124/mol.105.022046. PMID 16608920.
- ^ Najm, Fadi J.; Madhavan, Mayur; Zaremba, Anita; Shick, Elizabeth; Karl, Robert T.; Factor, Daniel C.; Miller, Tyler E.; Nevin, Zachary S.; Kantor, Christopher (2015-01-01).“Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo”. Nature. 522 (7555). doi:10.1038/nature14335.
- ^ United States Patent 5461068
External links
Medical
- Micatin
- Miconazole (National Institutes of Health)
- United States Patent 5461068 Imidazole derivative tincture and method of manufacture
Photographic
- Kodak process E6 Ektachrome (color transparency) processing manual Z-119
- Kodak process E6 Q-LAB processing manual Z-6 (more details than processing manual Z119 above)
| Systematic (IUPAC) name | |
|---|---|
|
(RS)-1-(2-(2,4-Dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole
|
|
| Clinical data | |
| Trade names | Desenex, Monistat, Zeasorb-AF |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601203 |
| Pregnancy category |
|
| Routes of administration |
topical, vaginal, sublabial,oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | n/a |
| Biological half-life | n/a |
| Excretion | n/a |
| Identifiers | |
| CAS Number | 22916-47-8 |
| ATC code | A01AB09 (WHO)A07AC01 (WHO)D01AC02 (WHO)G01AF04 (WHO)J02AB01 (WHO)S02AA13 (WHO) |
| PubChem | CID 4189 |
| IUPHAR/BPS | 2449 |
| DrugBank | DB01110 |
| ChemSpider | 4044 |
| UNII | 7NNO0D7S5M |
| KEGG | D00416 |
| ChEBI | CHEBI:6923 |
| ChEMBL | CHEMBL91 |
| Chemical data | |
| Formula | C18H14Cl4N2O |
| Molar mass | 416.127 g/mol |
| Chirality | Racemic mixture |

DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
///////////