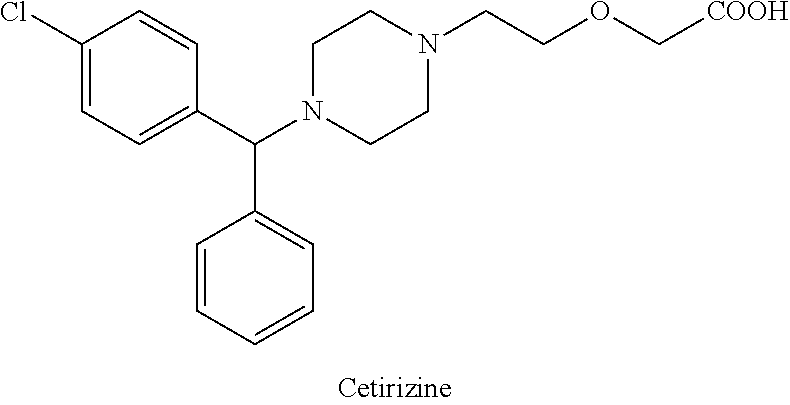
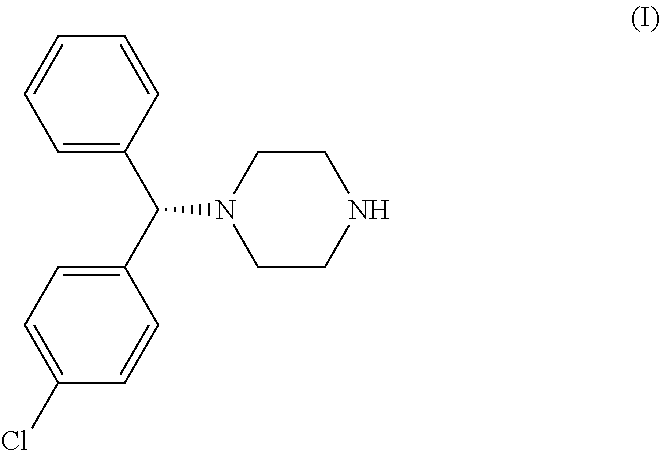
On August 20, 2013, Novartis announced in a press release that the FDA had granted breakthrough therapy designation to its experimental agent BYM338 (bimagrumab) for treatment of the rare muscle wasting disease sporadic inclusion body myositis (sIBM).
sIBM is a rare–but increasingly prevalent–disease. It is the most common cause of inflammatory myopathy in people over 50. sIBM has a yearly incidence of 2 to 5 per million adults with a peak at ages 50 to 70, with male predominance. Muscle wasting caused by sIBM is superimposed upon the sarcopenia (degenerative loss of muscle mass) that typically occurs with aging.
read all
bimagrumab
immunoglobulin G1-lambda2, anti-[Homo sapiens ACVR2B (activin
A receptor type IIB, ActR-IIB)], Homo sapiens monoclonal antibody;
gamma1 heavy chain (1-445) [Homo sapiens VH (IGHV1-2*02
(91.80%) -(IGHD)-IGHJ5*01 [8.8.8] (1-115) -IGHG1*03 (CH1 (116-
213), hinge (214-228), CH2 L1.3>A (232), L1.2>A (233) (229-338),
CH3 (339-443), CHS (444-445)) (116-445)], (218-216′)-disulfide with
lambda light chain (1′-217′) [Homo sapiens V-LAMBDA (IGLV2-
23*02 (90.90%) -IGLJ2*01) [9.3.11] (1′-111′) -IGLC2*01 (112′-217′)];
dimer (224-224”:227-227”)-bisdisulfide
myostatin inhibitor
bimagrumab immunoglobuline G1-lambda2, anti-[Homo sapiens ACVR2B
(récepteur type IIB de l’activine A, ActR-IIB)], Homo sapiens
anticorps monoclonal;
chaîne lourde gamma1 (1-445) [Homo sapiens VH (IGHV1-2*02
(91.80%) -(IGHD)-IGHJ5*01 [8.8.8] (1-115) -IGHG1*03 (CH1 (116-
213), charnière (214-228), CH2 L1.3>A (232), L1.2>A (233) (229-
338), CH3 (339-443), CHS (444-445)) (116-445)], (218-216′)-
disulfure avec la chaîne légère lambda (1′-217′) [Homo sapiens
V-LAMBDA (IGLV2-23*02 (90.90%) -IGLJ2*01) [9.3.11] (1′-111′) –
IGLC2*01 (112′-217′)]; dimère (224-224”:227-227”)-bisdisulfure
inhibiteur de la myostatine
inmunoglobulina G1-lambda2, anti-[Homo sapiens ACVR2B
(receptor tipo IIB de la activina A, ActR-IIB)], anticuerpo monoclonal
de Homo sapiens;
cadena pesada gamma1 (1-445) [Homo sapiens VH (IGHV1-2*02
(91.80%) -(IGHD)-IGHJ5*01 [8.8.8] (1-115) -IGHG1*03 (CH1 (116-
213), bisagra (214-228), CH2 L1.3>A (232), L1.2>A (233) (229-338),
CH3 (339-443), CHS (444-445)) (116-445)], (218-216′)-disulfuro con
la cadena ligera lambda (1′-217′) [Homo sapiens V-LAMBDA
(IGLV2-23*02 (90.90%) -IGLJ2*01) [9.3.11] (1′-111′) -IGLC2*01
(112′-217′)]; dímero (224-224”:227-227”)-bisdisulfuro
inhibidor de la miostatina
1356922-05-8
Heavy chain / Chaîne lourde / Cadena pesada
QVQLVQSGAE VKKPGASVKV SCKASGYTFT SSYINWVRQA PGQGLEWMGT 50
INPVSGSTSY AQKFQGRVTM TRDTSISTAY MELSRLRSDD TAVYYCARGG 100
WFDYWGQGTL VTVSSASTKG PSVFPLAPSS KSTSGGTAAL GCLVKDYFPE 150
PVTVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV 200
NHKPSNTKVD KRVEPKSCDK THTCPPCPAP EAAGGPSVFL FPPKPKDTLM 250
ISRTPEVTCV VVDVSHEDPE VKFNWYVDGV EVHNAKTKPR EEQYNSTYRV 300
VSVLTVLHQD WLNGKEYKCK VSNKALPAPI EKTISKAKGQ PREPQVYTLP 350
PSREEMTKNQ VSLTCLVKGF YPSDIAVEWE SNGQPENNYK TTPPVLDSDG 400
SFFLYSKLTV DKSRWQQGNV FSCSVMHEAL HNHYTQKSLS LSPGK 445
Light chain / Chaîne légère / Cadena ligera
QSALTQPASV SGSPGQSITI SCTGTSSDVG SYNYVNWYQQ HPGKAPKLMI 50
YGVSKRPSGV SNRFSGSKSG NTASLTISGL QAEDEADYYC GTFAGGSYYG 100
VFGGGTKLTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT 150
VAWKADSSPV KAGVETTTPS KQSNNKYAAS SYLSLTPEQW KSHRSYSCQV 200
THEGSTVEKT VAPTECS 217
Disulfide bridges location / Position des ponts disulfure / Posiciones de los puentes disulfuro
Intra-H 22-96 142-198 259-319 365-423
22”-96” 142”-198” 259”-319” 365”-423”
Intra-L 22′-90′ 139′-198′
22”’-90”’ 139”’-198”’
Inter-H-L 218-216′ 218”-216”’
Inter-H-H 224-224” 227-227”
N-glycosylation sites / Sites de N-glycosylation / Posiciones de N-glicosilación
H CH2 N84.4
Bimagrumab
http://www.who.int/medicines/publications/druginformation/innlists/PL108_Final.pdf
Novartis announced that the US Food and Drug Administration (FDA) has granted breakthrough therapy designation to BYM338 for sporadic inclusion body myositis (sIBM). This designation is based on the results of a phase 2 proof-of-concept study that showed BYM338 substantially benefited patients with sIBM compared to placebo.
read all at
Novartis receives FDA breakthrough therapy designation for BYM338 (bimagrumab) for sporadic inclusion body myositis (sIBM)
• Designation highlights potential of BYM338 to address an unmet medical need in a serious disease
• If approved, BYM338 has the potential to be the first treatment for sIBM patients
• BYM338 is the third Novartis investigational treatment this year to receive a breakthrough therapy designation by the FDA, highlighting Novartis’ leadership in the industry in breakthrough therapy designations
Bimagrumab (BYM338) is a human monoclonal antibody developed by Novartis to treat pathological muscle loss and weakness. On August 20, 2013 it was announced that bimagrumab was granted breakthrough therapy designation for sporadic inclusion body myositis(sIBM) by US Food and Drug Administration.[1]
- “Novartis receives FDA breakthrough therapy designation for BYM338 (bimagrumab) for sporadic inclusion body myositis (sIBM)”. Retrieved 20 August 2013.
World Health Organization (2012). “International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 108″ (PDF). WHO Drug Information 26(4)