liraglutide
read all at
http://www.pharmalive.com/novo-nordisk-says-will-seek-approval-of-obesity-drug-next-year
Systematic (IUPAC) name
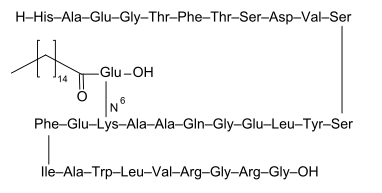
L-histidyl-L-alanyl-L-α-glutamylglycyl-L-threonyl-L-phenylalanyl-L-threonyl-L-seryl-L-α-aspartyl-L-valyl-L-seryl-L-seryl-L-tyrosyl-L-leucyl-L-α-glutamylglycyl-L-glutaminyl-L-alanyl-L-alanyl-N6-[N-(1-oxohexadecyl)-L-γ-glutamyl]-L-lysyl-L-α-glutamyl-L-phenylalanyl-L-isoleucyl-L-alanyl-L-tryptophyl-L-leucyl-L-valyl-L-arginylglycyl-L-arginyl-glycine
Liraglutide (NN2211), marketed under the brand name Victoza, is a long-acting glucagon-like peptide-1 agonist (GLP-1 agonist) developed by Novo Nordisk for the treatment of type 2 diabetes. The product was approved by the European Medicines Agency (EMA) on July 3, 2009, and by the U.S. Food and Drug Administration (FDA) on January 25, 2010.
Liraglutide is marketed under the brandname Victoza in the U.S., India, Canada, Europe and Japan. It has been launched in Germany, Denmark, the Netherlands, the United Kingdom, Ireland, Sweden, Japan, Canada, the United States, France, Malaysia and Singapore.
Phase I trials of an oral variant of Victoza (NN9924) started in 2010.