Itraconazole has been found to possess potent antiangiogenic activity, exhibiting promising antitumor activity in several human clinical studies. The wider use of itraconazole in the treatment of cancer, however, has been limited by its potent inhibition of the drug metabolizing enzyme cytochrome P450 3A4 (CYP3A4). In an effort to eliminate the CYP3A4 inhibition while retaining its antiangiogenic activity, we designed and synthesized a series of derivatives in which the 1,2,4-triazole ring is replaced with various azoles and nonazoles. Among these analogues, 15n with tetrazole in place of 1,2,4-triazole exhibited optimal inhibition of human umbilical vein endothelial cell proliferation with an IC50 of 73 nM without a significant effect on CYP3A4 (EC50 > 20 μM). Similar to itraconazole, 15n induced Niemann-Pick C phenotype (NPC phenotype) and blocked AMPK/mechanistic target of rapamycin signaling. These results suggest that 15n is a promising angiogenesis inhibitor that can be used in combination with most other known anticancer drugs.
Novel Tetrazole-Containing Analogues of Itraconazole as Potent Antiangiogenic Agents with Reduced Cytochrome P450 3A4 Inhibition
https://pubs.acs.org/doi/10.1021/acs.jmedchem.8b01252
■ ASSOCIATED CONTENT *S Supporting Information The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01252. Molecular formula strings (CSV) Detail of synthesis procedures; kinetic curve of CYP3A4 enzyme activities; philipin staining of compound 15c, 15g; competition assay of itraconazole photoaffinity probe; and NMR and HPLC chart of representative compounds (PDF)
■ AUTHOR INFORMATION Corresponding Author *E-mail: joliu@jhu.edu. Phone 410-955-4619. Fax 410-955- 4520. ORCID Wei Q. Shi: 0000-0001-5453-1753 Jun O. Liu: 0000-0003-3842-9841 Author Contributions § Y.L. and K.K.P. contributed equally to this work. Notes The authors declare no competing financial interest.
■ ACKNOWLEDGMENTS This work was supported by the National Cancer Institutes (grant R01CA184103) and the Flight Attendant Medical Research Institute
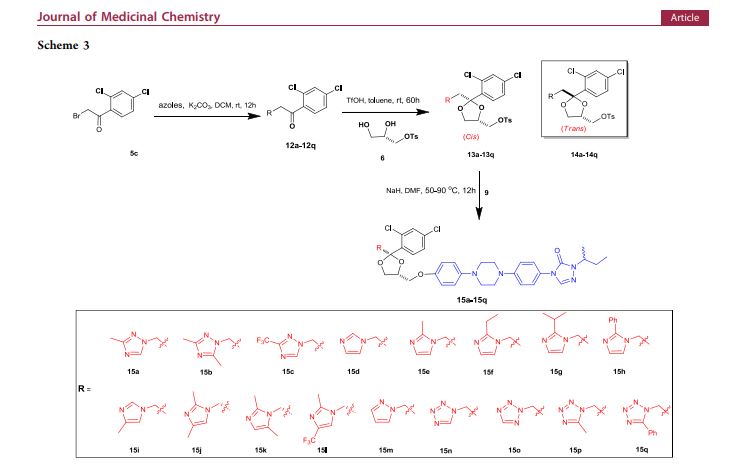
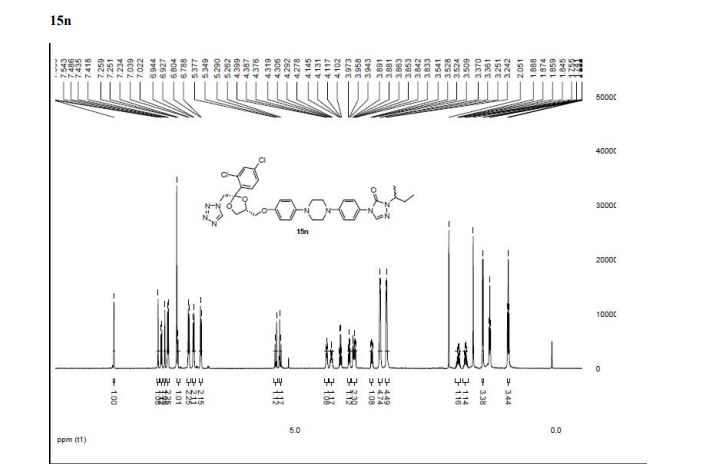
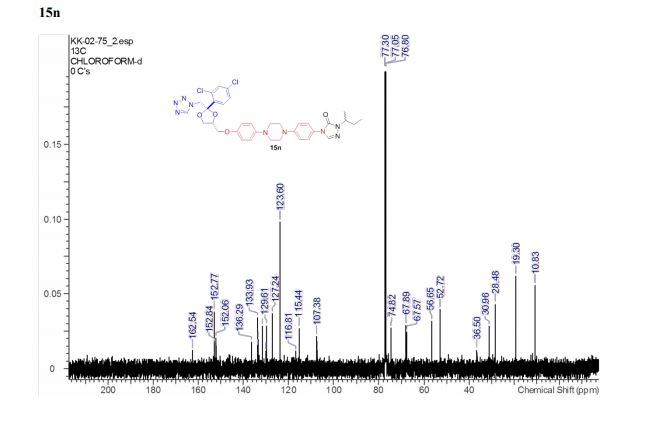
4-(4-(4-(4-(((2S,4R)-2-((1H-Tetrazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)phenyl)- 1-sec-butyl-1H-1,2,4-triazol-5(4H)-one (15n).
1 H NMR (500 MHz, CDCl3, δH): 8.46 (s, 1H), 7.61 (s, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.48 (d, J = 2.0 Hz, 1H), 7.43 (d, J = 9 Hz, 2H), 7.24 (dd, J = 8.5, 2.0 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 6.81 (d, J = 9.0 Hz, 2H), 5.36 (d, J = 14.0 Hz, 1H), 5.27 (d, J = 14.0 Hz, 1H), 4.38 (t, J = 5.0 Hz, 1H), 4.31−4.27 (m, 1H), 3.95 (dd, J = 8.5, 6.5 Hz, 1H), 3.88−3.83 (m, 2H), 3.53 (dd, J = 9.5, 6.5 Hz, 1H), 3.38 (br s, 4H), 3.26 (br s, 4H), 1.89−1.83 (m, 1H), 1.74−1.69 (m, 1H), 1.39 (d, J = 7.0 Hz, 3H), 0.90 (t, J = 7.5 Hz, 3H).
13C NMR (125 MHz, CDCl3, δC): 162.5, 152.8, 152.7, 152.0, 136.3, 133.9, 133.3, 131.5, 130.1, 129.6, 127.2, 123.6, 116.8, 115.4, 107.4, 74.8, 67.9, 67.6, 56.6, 52.7, 36.5, 31.0, 28.5, 19.2, 10.8.
HRMS (ESI) calcd for C34H37Cl2N9O4, 706.2424; found, 706.2425.
HPLC purity: 95.9%, tR = 10.5 min
-
Research Fellow at Nanyang Technological University, Singapore
-
Former Post-Doctoral Fellow at Johns Hopkins School of Medicine
-
Studied Physical organic chemistry at PG College of Science, Saifabad/Osmania University
-
Studied M.P.C at Government Junior College, Hanamkonda
-
Studied B.Sc. at Kakatiya Government Degree College
-
Went to Z. P. S. S. Geesugonda
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////