Elemental Impurities
On January 1, 2018, new guidelines regarding elemental impurities in brand and generic drug products went into effect. Elemental impurities, such as arsenic and lead, pose toxicological risks to patients without providing any therapeutic benefit. These impurities may be present in drug products from a variety of sources, such as interactions with equipment during the drug manufacturing process.
FDA, together with other organizations, such as the International Council for Harmonisation (ICH) and the U.S. Pharmacopeial Convention (USPC), have engaged in long-standing efforts to best protect patients from the risks posed by elemental impurities by developing limits for their amounts in drug products, and standardized approaches to use in determining the amount of elemental impurities in these products.
As of January 1, 2018:
- All new and existing NDAs and ANDAs for drug products with an official USP monograph are required to meet the requirements in USP General Chapters <232> and <233> for the control of elemental impurities.
- Applicants submitting NDAs and ANDAs for drug products without a USP monograph are expected to follow the recommendations in the ICH Q3D Elemental Impurities
 guideline.
guideline.
Questions and Answers on Elemental Impurities:
Why were these guidelines developed, and why are they important?
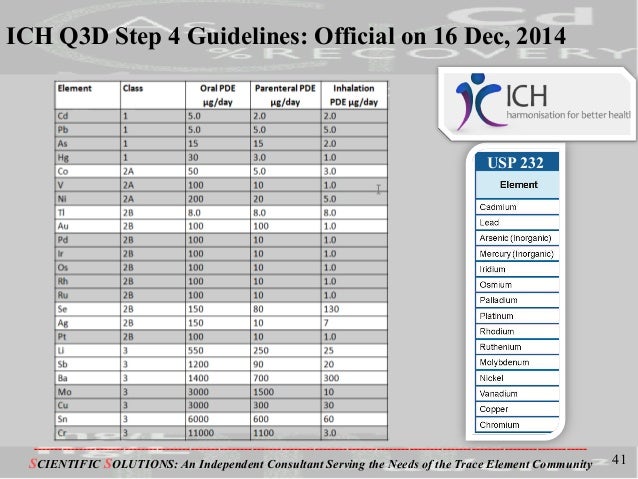
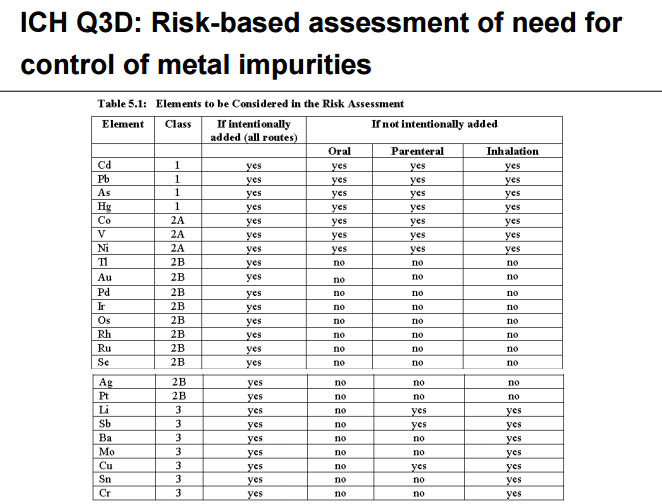
Heavy metal elemental impurities pose serious risks to patients without providing a benefit. Modern methods provide better analytical tests to detect elemental impurities, which in turn, will help protect patients by ensuring approved products have safe levels of these impurities. The ICH guidelines and USP General Chapters <232>Elemental Impurities—Limits are focused on establishing Permitted Daily Exposures (PDEs) for elemental impurities in drug products. USP General Chapter <233>Elemental Impurities—Procedures describes analytical approaches for the detection of elemental impurities. The analytical approaches described in <233> are based on modern analytical capabilities, replace the outdated tests in the deleted USP General Chapter <231> Heavy Metals, and allow us to more precisely measure impurities to ensure safe levels. FDA, ICH, USP, and industry experts worked together to develop the new standards that are in alignment and help ensure high quality medicines.
How has FDA been supporting industry to implement the requirements?
FDA, ICH, and USP have all engaged with brand and generic drug manufacturers to support implementation of these requirements. These requirements are the result of long-standing efforts, and both ICH and USP included industry participants on their expert panels that developed these standards. With that input, an implementation date was identified that provided firms with substantial time to verify their operations met the requirements.
In June 2016, FDA published a draft guidance, Elemental Impurities in Drug Products, to provide recommendations regarding the control of elemental impurities of human drug products. The draft guidance encouraged the early adoption of ICH Q3D guidelines and USP General Chapters <232> and <233> before the January 1, 2018 implementation date. FDA has also presented on this topic at conferences, including at a two-day ICH Q3D regional workshop it hosted in August 2016 1. These outreach efforts have supported efforts by industry to perform the risk assessments needed to implement the new guidelines in order to have complete, approvable applications. On an application-specific level, FDA began noting this requirement in complete response letters to applicants that contained quality deficiencies in Spring of 2017.
What should companies do if they have questions about elemental impurity standards?
Companies that have quality questions regarding elemental impurities and their applications should contact the Regulatory Business Process Manager (RBPM) in the Office of Program and Regulatory Operations, Office of Pharmaceutical Quality for their application. Applications that do not meet the elemental impurity guidelines are unable to be approved and applicants may receive a request for the information from the FDA in the form of an Information Request or a Complete Response letter. Firms should submit information on their elemental impurity risk assessments to FDA as soon as they are able, rather than waiting for a request from FDA, in order to minimize the impact on review and approval timeframes. The following resource may help applicants understand the process moving forward depending on where they are in the review process.
What is the International Council for Harmonisation?
ICH, first created in 1990 by regulatory agencies and both brand and generic drug manufacturing associations from the United States, Europe, and Japan, was established to facilitate international collaboration, and has been successful in standardizing and elevating drug development practices throughout the world. ICH’s mission helps to increase patient access to safe, effective, and high quality pharmaceuticals, and to ensure that pharmaceuticals are developed and registered efficiently. International harmonization of regulatory standards means that pharmaceutical manufacturers and developers will be held to the same standards in different markets (countries), which will make the development and delivery of quality pharmaceuticals to the public more timely and efficient. The ICH Website includes training modules on implementation of the Q3D elemental impurity guidelines.
What is the U.S. Pharmacopeia Convention?
The United States Pharmacopeia Convention (USPC) is a private non-profit organization that develops public standards related to pharmaceutical quality. USP General Chapters <232>Elemental Impurities—Limits, and, <233>Elemental Impurities—Procedures are applicable to compendial drug products as per Federal Food, Drug, and Cosmetic Act Sec. 201(j), and Sec. 501(b). USP’s website offers information regarding the history of actions they have taken on elemental impurities![]() , as well as other FAQ
, as well as other FAQ![]() .
.
1 Other presentations include the Drug Information Association’s CMC Workshop 2015![]() , the Consumer Healthcare Products Association’s 2015 Regulatory, Scientific & Quality Conference
, the Consumer Healthcare Products Association’s 2015 Regulatory, Scientific & Quality Conference![]() , the Product Quality Research Institute (PQRI) / USP Workshop on ICH Q3D Elemental Impurities Requirements
, the Product Quality Research Institute (PQRI) / USP Workshop on ICH Q3D Elemental Impurities Requirements![]() , the Generic Pharmaceutical Association (now Association of Affordable Medicines) CMC Workshop
, the Generic Pharmaceutical Association (now Association of Affordable Medicines) CMC Workshop![]() , the USP Excipients Stakeholder Forum, the PQRI/USP Workshop on Implementation Status of ICH Q3D
, the USP Excipients Stakeholder Forum, the PQRI/USP Workshop on Implementation Status of ICH Q3D![]() , and the PQRI/USP Workshop on ICH Q3D Elemental Impurities Requirements – Recent Experience and Plans for Full Implementation in 2018
, and the PQRI/USP Workshop on ICH Q3D Elemental Impurities Requirements – Recent Experience and Plans for Full Implementation in 2018![]()
Elemental Impurities
Efforts in this area are currently focused on three fronts:
-
Finalization of risk assessments to ensure compliance with the ICH Q3D guideline for all products supplied to those markets having implemented ICH Q3D and to the date for implementation
-
Continued development of ICH Q3D dermal limits
-
Removal of the heavy metals limit test USP <231>

Marketed Product Compliance
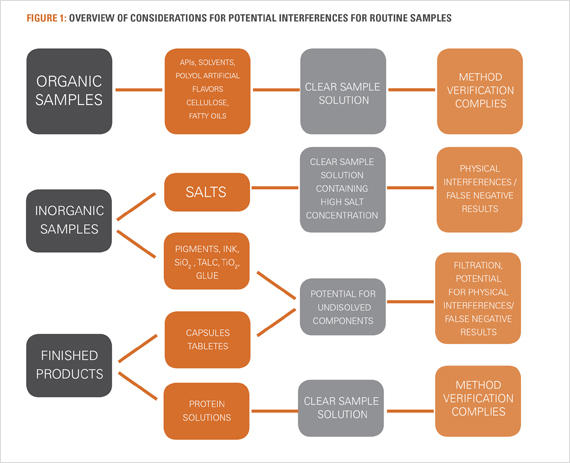
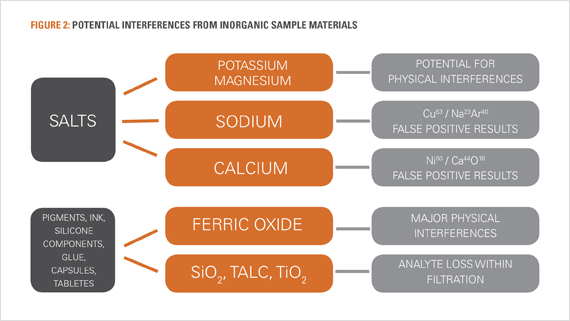
Elemental Impurities within Excipients

.png)
Removal of Heavy Metals Testing















.jpg)