DOI: 10.1039/C8GC00042E, Communication
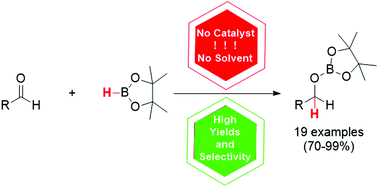
For the first time, a general method for catalyst-free and solvent-free hydroboration of various aldehydes has been developed
Catalyst-free and solvent-free hydroboration of aldehydes
Abstract
A simple catalyst-free and solvent-free method for the hydroboration of various aldehydes bearing a wide array of electron-withdrawing and electron-donating groups was developed. Unlike aldehydes, the addition of boranes to ketones is less efficient and is thus advantageous for the chemoselective reduction of the former ones. It is suggested that the described transformation proceeds with the formation of Lewis acid–base adducts, which facilitates further hydroboration.
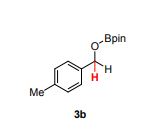
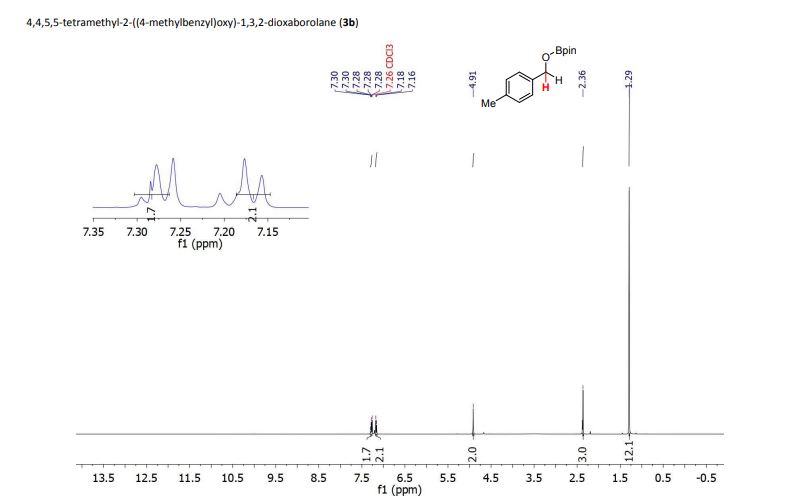
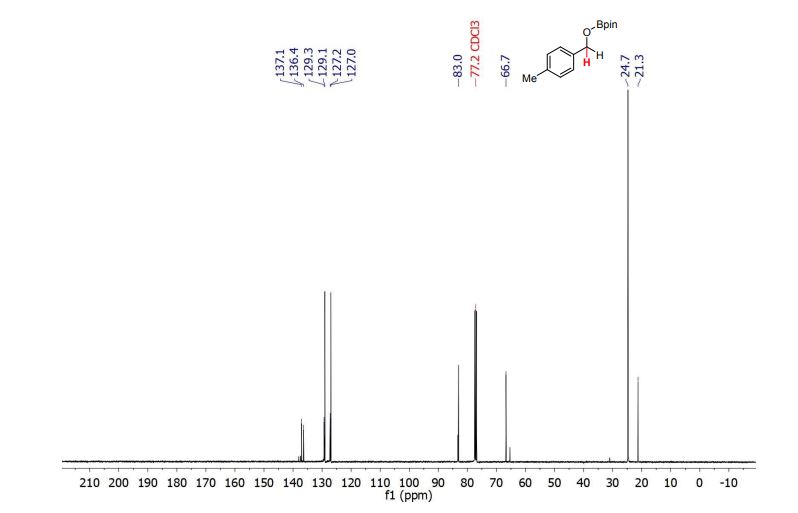
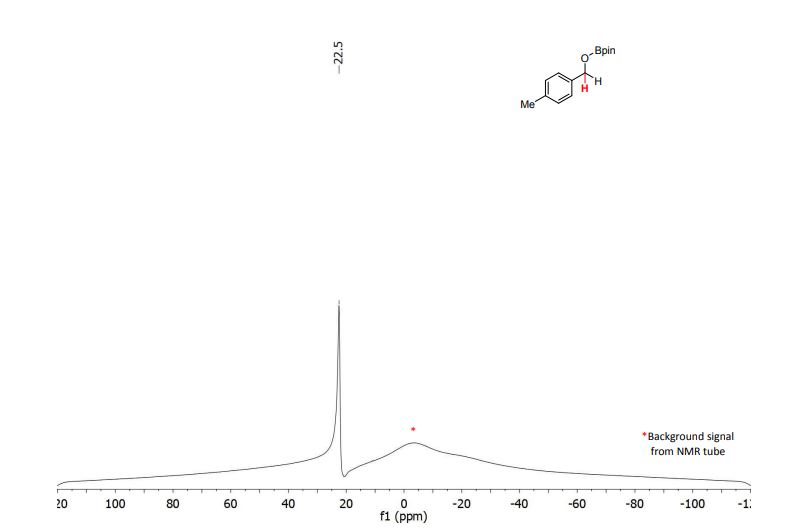
4,4,5,5-tetramethyl-2-((4-methylbenzyl)oxy)-1,3,2-dioxaborolane (3b) [1] 4,4,5,5-tetramethyl-2-((4-methylbenzyl)oxy)-1,3,2-dioxaborolane was obtained as colorless oil in 97% yield.
1H NMR (400 MHz, CDCl3) δ (ppm) = 1.29 (s, 12H), 2.36 (s, 3H), 4.91 (s, 2H), 7.17 (d, J = 7.9 Hz, 2H), 7.27-7.30 (m, 2H).
13C NMR (101 MHz, CDCl3) δ (ppm) = 21.3, 24.7, 66.7, 83.0, 127.0, 127.2, 129.1, 129.3, 136.4, 137.1.
11B NMR (128 MHz, CDCl3) δ (ppm) = 22.5.
EA: C14H21BO3 (248.16): calcd.C 67.77; H 8.53; found C 67.66; H 8.47.
11B NMR (128 MHz, CDCl3) δ (ppm) = 22.5.
////////////hydroboration