GSK 1070916
NMI-900 , GSK-1070916, GSK-1070916A
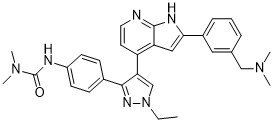
4-[3-(4-N,N-Dimethylcarbamylaminophenyl)-1-ethyl-1H-pyrazol-4-yl]-2-[3-(dimethylaminomethyl)phenyl]-1H-pyrrolo[2,3-b]pyridine
N’-[4-[4-[2-[3-[(Dimethylamino)methyl]phenyl]-1H-pyrrolo[2,3-b]pyridin-4-yl]-1-ethyl-1H-pyrazol-3-yl]phenyl]-N,N-dimethylurea
CAS 942918-07-2,
MFC30H33N7O,
MW507.63
PHASE 1/II , Advanced solid tumor, Cancer Research Technology,
off-white solid.
1H NMR (400 MHz, DMSO-d6) δ ppm 12.14 (d, J = 1.8 Hz, 1H), 8.31 (s, 1H), 8.27 (s, 1 H), 8.07 (d, J = 4.8 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.77 (s, 1H), 7.43 (d, J = 8.6 Hz, 2H), 7.39 (d, J = 8.1 Hz, 1H), 7.27 (d, J = 8.6 Hz, 2H), 7.27 (dd, 1H), 6.79 (d, J = 5.1 Hz, 1H), 6.76 (d, J = 2.0 Hz, 1H), 4.27 (q, J = 7.3 Hz, 2H), 3.43 (s, 2H), 2.91 (s, 6H), 2.18 (s, 6H), 1.51 (t, J = 7.2 Hz, 3H).
MS m/z 508.4 [M + H]+. Anal. (C30H33N7O·1.0H2O) C, H, N.
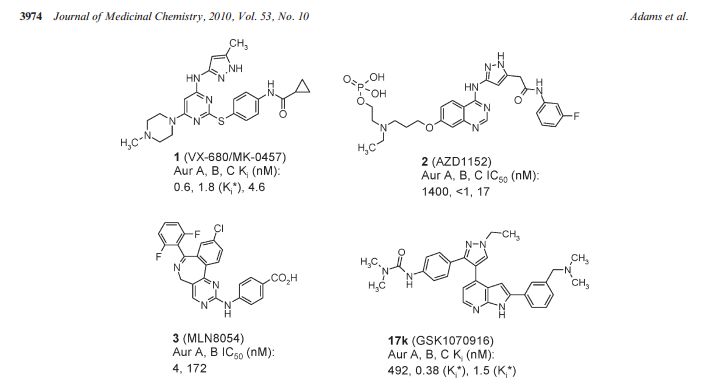
GSK1070916 is a reversible and ATP-competitive inhibitor of Aurora B/C with IC50 of 3.5 nM/6.5 nM; displays >100-fold selectivity against the closely related Aurora A-TPX2 complex(IC50=490 nM).
NMI-900, an Aurora B/C kinase inhibitor, is under development at Cancer Research Technology in phase I/II clinical studies for the treatment of advanced and/or metastatic solid tumors. Other phase I clinical trials for the treatment of solid tumors had been previously completed, in a collaboration between GlaxoSmithKline and Cancer Research Technology, under the Cancer Research UK’s Clinical Development Partnerships (CDP) program.
The drug was originated by GlaxoSmithKline. The rights of the product were acquired by Cancer Research Technology from GlaxoSmithKline after the company elected not to take the program forward. In December 2015, the product was licensed by Cancer Research Technology to Nemucore Medical Innovations for the exclusive worldwide development and commercialization for the treatment of difficult-to-treat cancers.

PATENT
US 20070149561
https://www.google.com/patents/US20070149561
PAPER
Journal of Medicinal Chemistry (2010), 53 (10), 3973-4001
http://pubs.acs.org/doi/abs/10.1021/jm901870q
Discovery of GSK1070916, a Potent and Selective Inhibitor of Aurora B/C Kinase

The Aurora kinases play critical roles in the regulation of mitosis and are frequently overexpressed or amplified in human tumors. Selective inhibitors may provide a new therapy for the treatment of tumors with Aurora kinase amplification. Herein we describe our lead optimization efforts within a 7-azaindole-based series culminating in the identification of GSK1070916 (17k). Key to the advancement of the series was the introduction of a 2-aryl group containing a basic amine onto the azaindole leading to significantly improved cellular activity. Compound 17k is a potent and selective ATP-competitive inhibitor of Aurora B and C with Ki* values of 0.38 ± 0.29 and 1.5 ± 0.4 nM, respectively, and is >250-fold selective over Aurora A. Biochemical characterization revealed that compound 17k has an extremely slow dissociation half-life from Aurora B (>480 min), distinguishing it from clinical compounds 1 and 2. In vitro treatment of A549 human lung cancer cells with compound 17k results in a potent antiproliferative effect (EC50 = 7 nM). Intraperitoneal administration of 17k in mice bearing human tumor xenografts leads to inhibition of histone H3 phosphorylation at serine 10 in human colon cancer (Colo205) and tumor regression in human leukemia (HL-60). Compound 17k is being progressed to human clinical trials.
http://pubs.acs.org/doi/pdf/10.1021/jm901870q………..PDF FILE
PAPER
Molecules 2014, 19(12), 19935-19979; doi:10.3390/molecules191219935
http://www.mdpi.com/1420-3049/19/12/19935/htm

http://www.mdpi.com/1420-3049/19/12/19935/htm
Biological Activity of GSK-1070916
GSK1070916 is a reversible and ATP-competitive inhibitor of Aurora B/C with IC50 of 3.5 nM/6.5 nM; displays >100-fold selectivity against the closely related Aurora A-TPX2 complex(IC50=490 nM).
IC50 Value: 3.5 nM(Aurora B); 6.5 nM(Aurora C)
Target: Aurora B/C
in vitro: GSK1070916 selectively inhibits Aurora B and Aurora C with Ki of 0.38 nM and 1.5 nM over Aurora A with Ki of 490 nM. Inhibition of Aurora B and Aurora C is time-dependent, with an enzyme-inhibitor dissociation half-life of >480 min and 270 min respectively. In addition, GSK1070916 is also a competitive inhibitor with respect to ATP. Human tumor cells treated with GSK1070916 shows dose-dependent inhibition of phosphorylation on serine 10 of Histone H3, a substrate specific for Aurora B. Moreover, GSK1070916 inhibits the proliferation of tumor cells with EC50 values of <10 nM in over 100 cell lines spanning a broad range of tumor types, with a median EC50 of 8 nM. Although GSK1070916 has potent activity against proliferating cells, a dramatic shift in potency is observed in primary, nondividing, normal human vein endothelial cells. Furthermore, GSK1070916-treated cells do not arrest in mitosis but instead fails to divide and become polyploid, ultimately leading to apoptosis. In another study, it is also reported high chromosome number associated with resistance to the inhibition of Aurora B and C suggests cells with a mechanism to bypass the high ploidy checkpoint are resistant to GSK1070916.
in vivo: GSK1070916 (25, 50, or 100 mg/kg) shows dose-dependent inhibition of phosphorylation of an Aurora B–specific substrate in mice and consistent with its broad cellular activity, has antitumor effects in 10 human tumor xenograft models including breast, colon, lung, and two leukemia models.
Clinical Information of GSK-1070916
| Product Name | Sponsor Only | Condition | Start Date | End Date | Phase | Last Change Date |
|---|---|---|---|---|---|---|
| GSK-1070916 | Cancer Research UK | Advanced solid tumor | 31-MAR-10 | 31-MAR-13 | Phase 1 | 17-JUN-13 |
References on GSK-1070916
/////////////GSK1070916, GSK-1070916, 942918-07-2 GSK, phase1, Advanced solid tumor, NMI-900 , GSK-1070916, GSK-1070916A