GDC-0084
CAS#: 1382979-44-3
Chemical Formula: C18H22N8O2
Exact Mass: 382.1866
Synonym: RG7666; RG-7666; RG 7666; GDC-0084; GDC0084; GDC 0084.
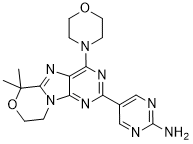
IUPAC/Chemical Name: 5-(6,6-dimethyl-4-morpholino-8,9-dihydro-6H-[1,4]oxazino[4,3-e]purin-2-yl)pyrimidin-2-amine
| Latest Stage of Development | Phase I |
| Standard Indication | Brain cancer |
| Indication Details | Treat progressive or recurrent high-grade glioma |
| Regulatory Designation | |
| Partner | Genentech Inc. |
- Originator Genentech
- Class Antineoplastics; Small molecules
- Mechanism of Action 1 Phosphatidylinositol 3 kinase inhibitors
- 28 Jan 2015 Discontinued – Phase-I for Glioma in Spain (unspecified route)
- 28 Jan 2015 Discontinued – Phase-I for Glioma in USA (unspecified route)
- 01 Jan 2015 Genentech completes a phase I trial in Glioma in USA and Spain (NCT01547546)
GDC-0084, also known as RG7666, is a phosphatidylinositol 3-kinase (PI3K) inhibitor with potential antineoplastic activity. PI3K inhibitor GDC-0084 specifically inhibits PI3K in the PI3K/AKT kinase (or protein kinase B) signaling pathway, thereby inhibiting the activation of the PI3K signaling pathway. This may result in the inhibition of both cell growth and survival in susceptible tumor cell populations. Activation of the PI3K signaling pathway is frequently associated with tumorigenesis.
http://pubs.acs.org/doi/pdf/10.1021/acsmedchemlett.6b00005

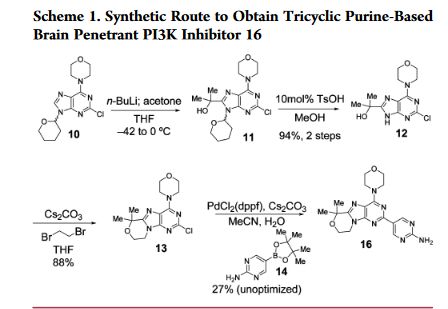
An improved, efficient process with a significantly reduced process mass intensity (PMI) led to the multikilogram synthesis of a brain penetrant PI3K inhibitor GDC-0084. Highlights of the synthesis include a phase transfer catalyzed annulation in water, an efficient Suzuki-Miyaura cross-coupling of a chloropyrimidine with an arylboronic acid using a low palladium catalyst loading, and the development of a controlled crystallization to provide the API. The process delivered GDC-0084 with low levels of both impurities and residual metals.
Development of an Efficient, Safe, and Environmentally Friendly Process for the Manufacture of GDC-0084
//////GDC-0084
NC1=NC=C(C2=NC(N3CCOCC3)=C4N=C(C(C)(C)OCC5)N5C4=N2)C=N1
5-(6,6-Dimethyl-4-morpholino-8,9-dihydro-6H-[1,4]oxazino[4,3-e]purin-2-yl)pyrimidin-2-amine GDC-0084
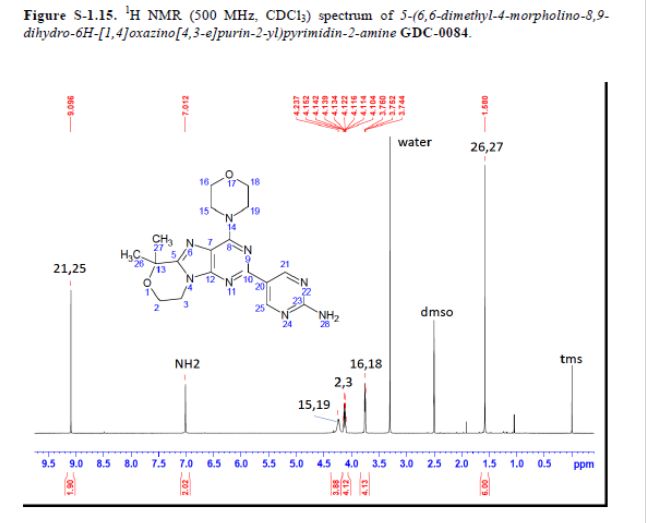
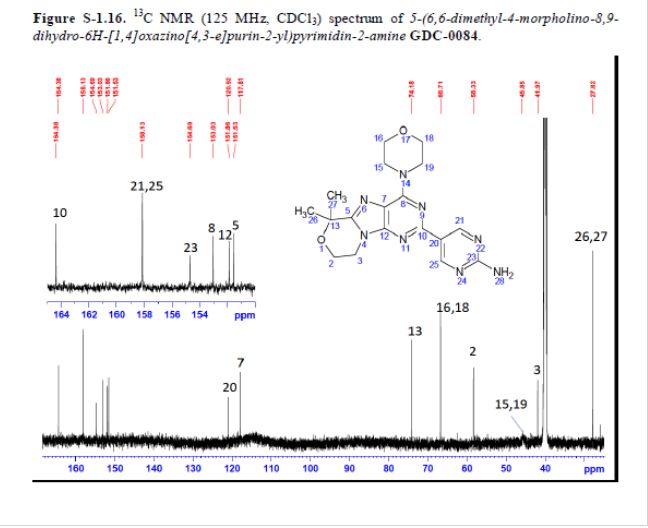
mp 211 °C; 1H NMR (500 MHz, DMSO-d6) δ 9.09 (s, 2H), 7.03 (s, 2H), 4.32–4.17 (m, 4H), 4.17–4.04 (m, 4H), 3.84–3.65 (m, 4H), 1.58 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 163.8, 157.6, 154.2, 152.5, 151.3, 151.0, 120.3, 117.3, 73.7, 66.2, 57.8, 45.2, 41.5, 27.3. HRMS [M + H]+calcd for C18H22N8O2 383.1938; found 383.1945.
-
The Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of Class I Phosphoinositide 3-Kinases (PI3K) and mTOR.
Heffron, T.; Ndubaku, C.; Salphati, L.; Alicke, B.; Cheong, J.;Drobnick, J.; Edgar, K.; Gould, S.; Lee, L.; Lesnick, J.; Lewis, C.; Nonomiya, J.; Pang, j.; Plise, E.; Sideris,S.; Wallin, J.; Wang, L.; Zhang, X.; Olivero, A. ACS Med. Chem. Lett. 2016, , DOI: 10.1021/acsmedchemlett.6b00005
-
(a) Purine Derivatives Useful as PI3 Kinase Inhibitors. Goldsmith, P.; Hancox, T. C.; Hudson, A.; Pegg, N. A.; Kulagowski, J. J.; Nadin, A. J.; Price, S. PCT Int. Appl. WO 2009053716 A1 Apr 30, 2009.
(b) Preparation of Purine Derivatives with PI3K Inhibitory Activity and Methods of Use Thereof. Castanedo, G.; Chuckowree,I.; Folkes, A.; Sutherlin, D. P.; Wan, N. C. PCT Int. Appl. WO 2009146406 A1 Dec 3, 2009