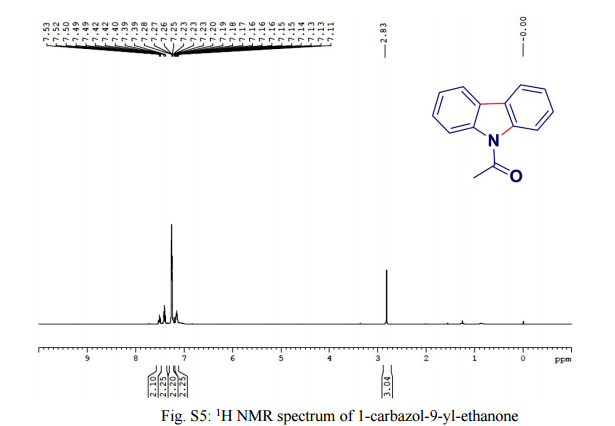
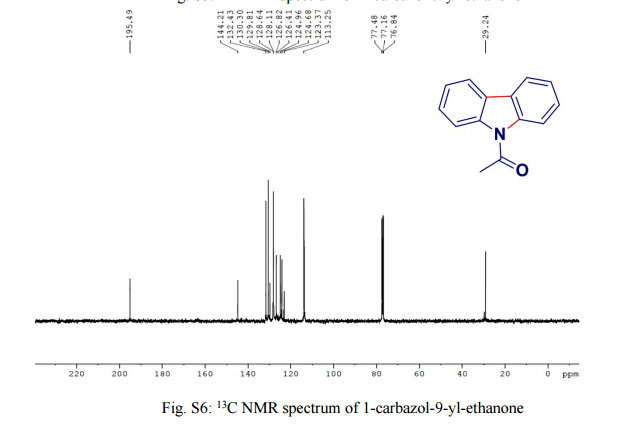
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02937F, Paper
DOI: 10.1039/C5GC02937F, Paper
Vignesh Arumugam, Werner Kaminsky, Dharmaraj Nallasamy
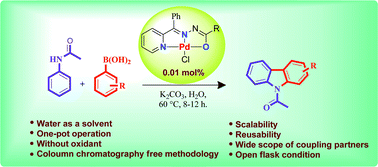
NNO Pincer type Pd(II) complex catalyzed one-pot synthesis of N-acetylcarbazoles in aqueous media is presented.
NNO Pincer type Pd(II) complex catalyzed one-pot synthesis of N-acetylcarbazoles in aqueous media is presented.
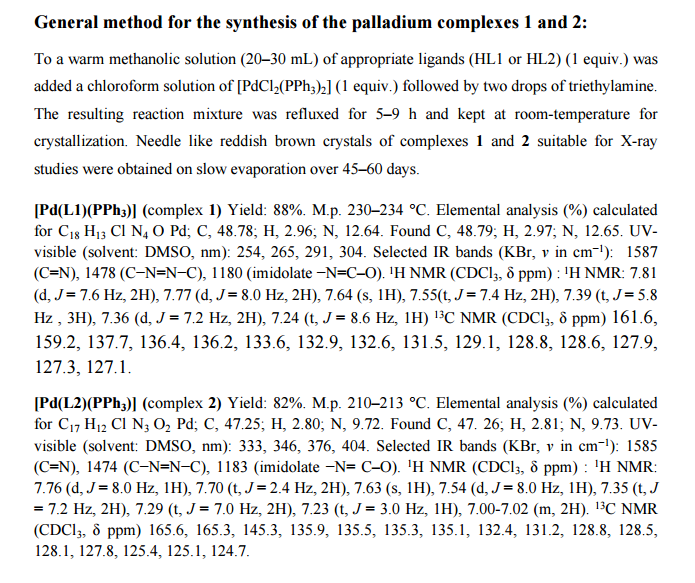
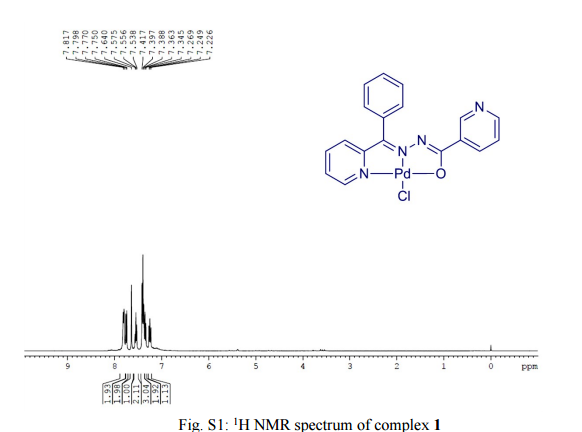
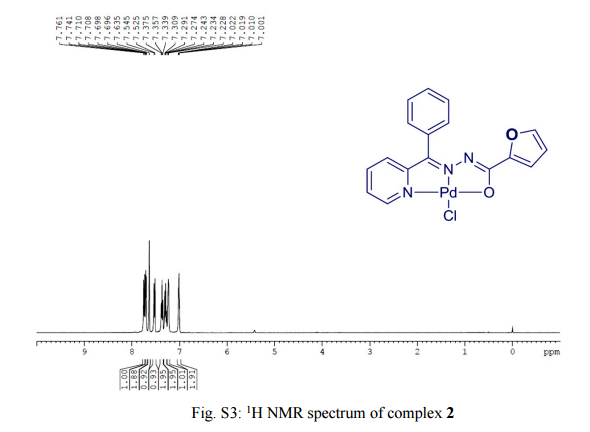
One-pot, tandem C–H and N–H activation of acetanilides with aryl boronic acids to realize functionalized carbazoles was conveniently performed under aerobic conditions using a novelNNO pincer type Pd(II) complex [Pd(L)Cl] (where L = nicotinic acid (phenyl-pyridin-2-yl-methylene)-hydrazide or furan-2-carboxylic acid (phenyl-pyridin-2-yl-methylene)-hydrazide) as a catalyst in neat water and a very low (0.01 mol%) amount of catalyst. It is worth noting that recyclability up to six consecutive runs and column chromatography free isolation of the title heterocycles in an excellent yield are achieved.
Pd(II) pincer type complex catalyzed tandem C–H and N–H activation of acetanilide in aqueous media: a concise access to functionalized carbazoles in a single step
*Corresponding authors
aInorganic & Nanomaterials Research Laboratory, Department of Chemistry, Bharathiar University, Coimbatore 641 046, India
E-mail: dharmaraj@buc.edu.in
Web: http://ndharmaraj.wix.com/inrl
Fax: +91 4222422387
Tel: +91 4222428316
E-mail: dharmaraj@buc.edu.in
Web: http://ndharmaraj.wix.com/inrl
Fax: +91 4222422387
Tel: +91 4222428316
bDepartment of Chemistry, University of Washington, Seattle, USA
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02937F
One-pot, tandem C–H and N–H activation of acetanilides with aryl boronic acids to realize functionalized carbazoles was conveniently performed under aerobic conditions using a novelNNO pincer type Pd(II) complex [Pd(L)Cl] (where L = nicotinic acid (phenyl-pyridin-2-yl-methylene)-hydrazide or furan-2-carboxylic acid (phenyl-pyridin-2-yl-methylene)-hydrazide) as a catalyst in neat water and a very low (0.01 mol%) amount of catalyst. It is worth noting that recyclability up to six consecutive runs and column chromatography free isolation of the title heterocycles in an excellent yield are achieved.
////////