Scheme 1: Flow synthesis of fluoxetine (46).
PIC CREDIT, The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry, and , Beilstein J. Org. Chem. 2015, 11, 1194–1219.,doi:10.3762/bjoc.11.134
One of the early published examples of industry-based research on multi-step flow synthesis of a pharmaceutical was reported in 2011 by scientists from Eli Lilly/UK and detailed the synthesis of fluoxetine 46, the API of Prozac[1]. In this account each step was performed and optimised individually in flow, with analysis and purification being accomplished off-line. The synthesis commences with the reduction of the advanced intermediate ketone 47 using a solution of pre-chilled borane–THF complex (48) to yield alcohol 49 (Scheme 1).
Conversion of the pendant chloride into iodide 51 was attempted via Finckelstein conditions, however, even when utilising phase-transfer conditions in order to maintain a homogeneous flow regime the outcome was not satisfactory giving only low conversions. Alternatively direct amination of chloride 49 utilising high temperature flow conditions (140 °C) allowed the direct preparation of amine 50 in excellent yield.
Flow processing using a short residence time (10 min) at the elevated temperature allowed for a good throughput; in addition, the handling of the volatile methylamine within the confines of the flow reactor simplifies the practical aspects of the transformation, however, extra precautions were required in order to address and remove any leftover methylamine that would pose a significant hazard during scaling up.
The final arylation of 50 was intended to be performed as a SNAr reaction, however, insufficient deprotonation of the alcohol 50 under flow conditions (NaHMDS or BEMP instead of using a suspension of NaH as used in batch) required a modification to the planned approach. To this end a Mitsunobu protocol based on the orchestrated mixing of four reagent streams (50, 54 and reagents 52 and 53) was developed and successfully applied to deliver fluoxetine (46) in high yield.
Overall, this study is a good example detailing the intricacies faced when translating an initial batch synthesis into a sequence of flow steps for which several adaptations regarding choice of reagents and reaction conditions are mandatory in order to succeed.
- Ahmed-Omer, B.; Sanderson, A. J. Org. Biomol. Chem. 2011, 9, 3854–3862. doi:10.1039/C0OB00906G
Paper
Preparation of fluoxetine by multiple flow processing steps
*Corresponding authorsaEli Lilly and Co. Ltd., Lilly Research Centre, Erl Wood Manor, Windlesham, Surrey, UKOrg. Biomol. Chem., 2011,9, 3854-3862DOI: 10.1039/C0OB00906G
http://pubs.rsc.org/en/Content/ArticleLanding/2011/OB/c0ob00906g#!divAbstract
Microflow technology is established as a modern and fashionable tool in synthetic organic chemistry, bringing great improvement and potential, on account of a series of advantages over flask methods. The study presented here focuses on the application of flow chemistry process in performing an efficient multiple step syntheses of (±)-fluoxetine as an alternative to conventional synthetic methods, and one of the few examples of total synthesis accomplished by flow technique.
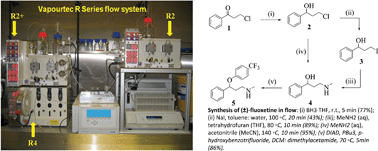
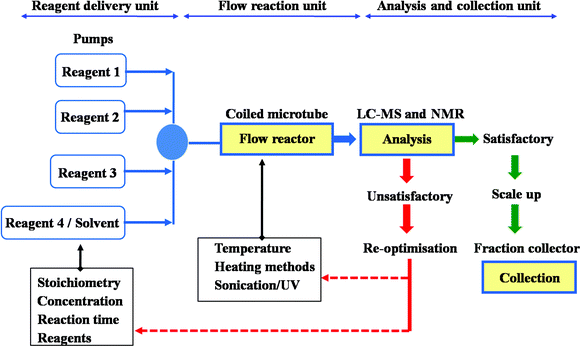
1 The general method set-up of flow process used for the synthesis of (±)- fluoxetine.
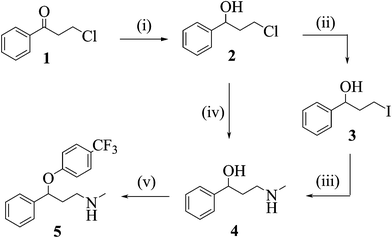
Scheme 1 Synthesis of (±)-fluoxetine in flow: (i) BH3·THF, r.t., 5 min (77%); (ii) NaI, toluene: water, 100 °C, 20 min (43%); (iii); MeNH2 (aq), …
//////////Flow synthesis, fluoxetine












![[1860-5397-11-134-i8]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-11-134-i8.png?max-width=550&background=EEEEEE)


 LinkedIn
LinkedIn Facebook
Facebook Twitter
Twitter GooglePlus
GooglePlus