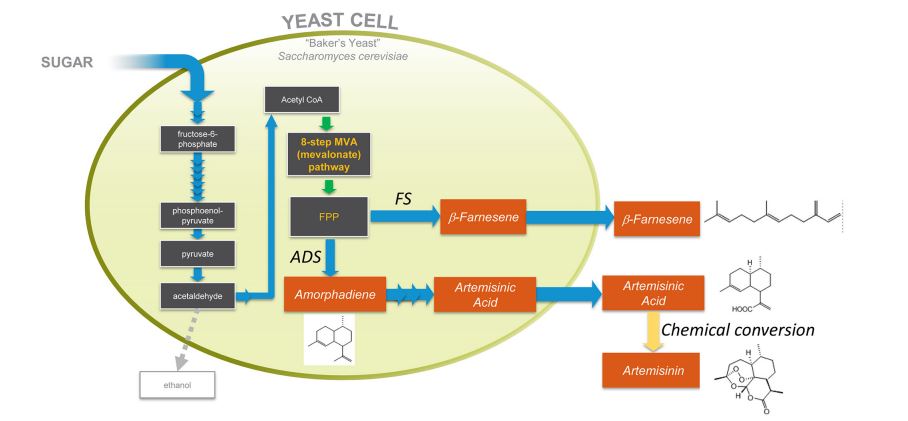
Figure 1. Production of artemisinic acid or β-farnesene by engineered yeast. The sesquiterpene alkenes β-farnesene and amorphadiene are both derived from FPP (farnesyl diphosphate) by the action of specific enzymes introduced from plants: amorphadiene synthase (ADS) generates amorphadiene and β-farnesene synthase (FS) generates β-farnesene. Production strains express either ADS or FS, not both. Oxidation of amorphadiene to artemisinic acid is accomplished by the action of five plant enzymes expressed in the engineered yeast.17 Conversion of purified artemisinic acid to artemisinin is accomplished by in vitro organic chemistry. Isoprenoid production strains make little ethanol.
The antimalarial drug artemisinin and the specialty chemical β-farnesene are examples of natural product isoprenoids that can help solve global challenges, but whose usage has previously been limited by supply and cost impediments. This review describes the path to commercial production of these compounds utilizing fermentation of engineered yeast. Development of commercially viable yeast strains was a substantial challenge that was addressed by creation and implementation of an industrial synthetic biology pipeline. Using the engineered strains, production of β-farnesene from Brazilian sugarcane offers several environmental advantages. Among the many commercial applications of β-farnesene, its use as a feedstock for making biodegradable lubricants is highlighted. This example, along with others, highlight a powerful new suite of technologies that will become increasingly important for production of chemicals, spanning from pharmaceuticals through commodity chemicals.
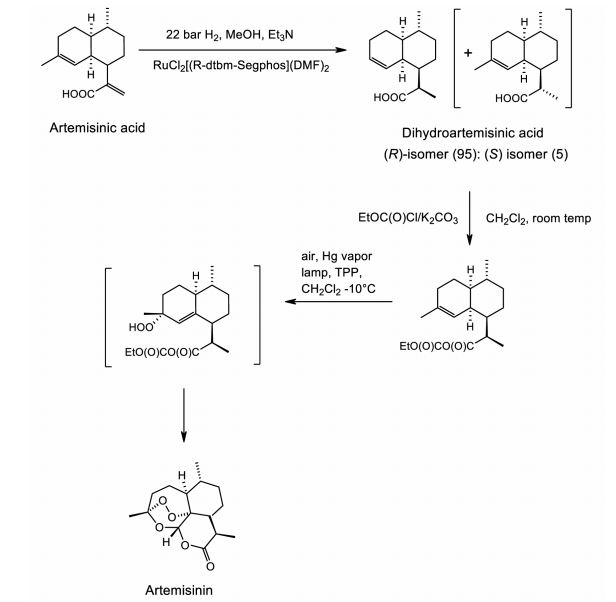
Figure 2. Sanofi industrial semi-synthesis of artemisinin. The process starts with a moderate pressure catalytic diastereoselective hydrogenation of artemisinic acid to produce a high (95:5) ratio of the desired (R)-isomer. To avoid formation of a lactone byproduct, dihydro-epi-deoxyarteannuin B, during the photooxidation, the carboxylic acid is protected as a mixed anhydride. The final step combines formation of the intermediate hydroperoxide via photoxidation using a Hg vapor lamp and commercially available tetraphenylporphyin (TPP) as sensitizer with a Hock cleavage and rearrangement catalyzed by trifluoroacetic acid to give, after workup, the best yield reported to date of pure isolated artemisinin (55%).
Synthetic Biology and the Development of Commercial β-Farnesene Production Strains Semi-synthetic artemisinin is a pharmaceutical with a price point comparable to plant-derived artemisinin,20 namely above $150 per kg. β-Farnesene, however, is a specialty chemical with multiple uses (more details below); most specialty and commodity chemicals have significantly lower price points, often below $10 per kg. For these product categories, it is of paramount importance that fermentative production be as efficient as possible, with high yields (namely, grams of product made per gram of feed substrate), productivities (grams of product/liter of culture/hour) and concentration (also known as titer; grams of product per liter of culture). Developing yeast strains capable of the yield, productivity and titer required for chemical production requires extensive development, and has been enabled over the last decade by the new discipline of synthetic biology. Synthetic biology seeks to extend approaches and concepts from engineering and computation to redesign biology for a chosen function;21recent advances in the application of design automation, i.e., the use of software, hardware and robotics22 have enabled the creation and screening of hundreds of thousands of strain variants (created by both design and random mutagenesis) for the properties required for commercial production of β-farnesene. Notable enabling technologies developed for routine usage include rapid and reliable assembly of large (i.e., multiple kilobase) deoxyribonucleic acid (DNA) constructs;23-25 high throughput, cost effective, verification of structural DNA assemblies by both initial restriction digest26 and by low-cost DNA sequencing;27 and whole genome sequencing of yeast strains.28 In addition, there is a need to effectively identify the best new strains (akin to panning for gold!) through high throughput, rapid, and accurate methods to screen thousands of strains. Further, the results of small-scale (< 1 milliliter) tests must correspond to the results of large-scale (> 50,000 liter) production. Development and implementation of these technologies required considerable investment by Amyris. The outcome is a robust pipeline for efficient, cost-effective strain generation allied with screening for the properties required for commercial production of β-farnesene by fermentation (i.e., at a price point required for its use as a specialty chemical).
As the world’s population and economies grow, the demand for a wide variety of specialty, commodity, and pharmaceutical chemicals will outpace the supply available from current sources. There is an urgent need to develop alternative, sustainable sources of many existing chemicals and to develop abundant sources of currently scarce chemicals with novel beneficial properties. Synthetic biology and industrial fermentation, combined with synthetic chemistry, will be an increasingly important source of chemicals in the decades ahead; artemisinin and β-farnesene provide good examples of this relatively new approach to chemical production. Brazil’s plentiful sugar cane feedstock and fermentation expertise make it an excellent location for this type of manufacturing, which can expand and diversify the nation’s industrial base and international importance.
J. Braz. Chem. Soc. 2016, 27(8), 1339-1345
Developing Commercial Production of Semi-Synthetic Artemisinin, and of β-Farnesene, an Isoprenoid Produced by Fermentation of Brazilian Sugar
Kirsten R. Benjamin; Iris R. Silva; João P. Cherubim; Derek McPhee; Chris J. Paddon
 Genes encoding the biosynthetic pathway for production of a valuable product (e.g., farnesene) in a native organism are expressed in a heterologous microbial host (e.g., yeast). The engineered yeast produces farnesene by commercial fermentation. Copyright © 2016 Amyris, inc. All rights reserved.
Genes encoding the biosynthetic pathway for production of a valuable product (e.g., farnesene) in a native organism are expressed in a heterologous microbial host (e.g., yeast). The engineered yeast produces farnesene by commercial fermentation. Copyright © 2016 Amyris, inc. All rights reserved.
http://dx.doi.org/10.5935/0103-5053.20160119
http://jbcs.sbq.org.br/imagebank/pdf/v27n8a04.pdf
Kirsten R. Benjamin,a Iris R. Silva,b João P. Cherubim,c Derek McPheea and Chris J. Paddon*,a a Amyris, Inc., 5885 Hollis Street, Suite 100, CA 94608 Emeryville, USA b Amyris Brasil Ltda, Rua John Dalton 301-Bloco B-Edificio 3, Condominio Techno Plaza, 13069-330 Campinas-SP, Brazil c Amyris Brasil Ltda, Rodovia Brotas/Torrinha-km 7.5, 17380-000 Brotas-SP, Brazil

Dr. Paddon has a PhD in Biochemistry from Imperial College, London, but now considers himself a synthetic biologist. After postdoctoral work at the National Institutes of Health in Bethesda, MD, he worked in the pharmaceutical industry (GlaxoSmithKline), and then for two Bay Area biopharmaceutical companies (Affymax and Xenoport) before joining Amyris, Inc. in 2005 as its sixth employee and first scientist. He was project leader for the semi-synthetic artemisinin project at Amyris, Inc. and has subsequently led a number of other projects and programs there.
//////////// Commercial Production, Semi-Synthetic , Artemisinin, farnesene, fermentation, natural product, lubricant