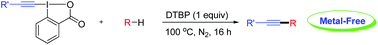Directed alkynylation of unactivated C(sp3)-H bonds with ethynylbenziodoxolones mediated by DTBP
Green Chem., 2016, 18,4185-4188
DOI: 10.1039/C6GC01336H, Communication
Zhi-Fei Cheng, Yi-Si Feng, Chun Rong, Tao Xu, Peng-Fei Wang, Jun Xu, Jian-Jun Dai, Hua-Jian Xu
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
A general and efficient alkynylation of unactivated C(sp3)-H bonds under metal-free conditions was developed herein.
Directed alkynylation of unactivated C(sp3)–H bonds with ethynylbenziodoxolones mediated by DTBP
*Corresponding authors
aSchool of Chemistry and Chemical Engineering, School of Biological and Medical Engineering, Hefei University of Technology, Hefei 230009, P. R. China
bAnhui Key Laboratory of Controllable Chemical Reaction and Material Chemical Engineering, Hefei 230009, P. R. China
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
E-mail: hjxu@hfut.edu.cn
Fax: (+86)-551-62904405
cAnhui Provincial Laboratory of Heterocyclic Chemistry, Maanshan 243110, China
Green Chem., 2016,18, 4185-4188
DOI: 10.1039/C6GC01336H, http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC01336H?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
A general and efficient method for the direct alkynylation of unactivated C(sp3)–H bonds under metal-free conditions is described. The reaction performs smoothly under mild conditions and shows excellent functional-group tolerance. Initial mechanistic investigation indicates that the reaction may involve a radical pathway.
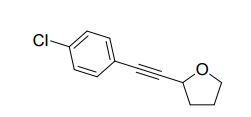
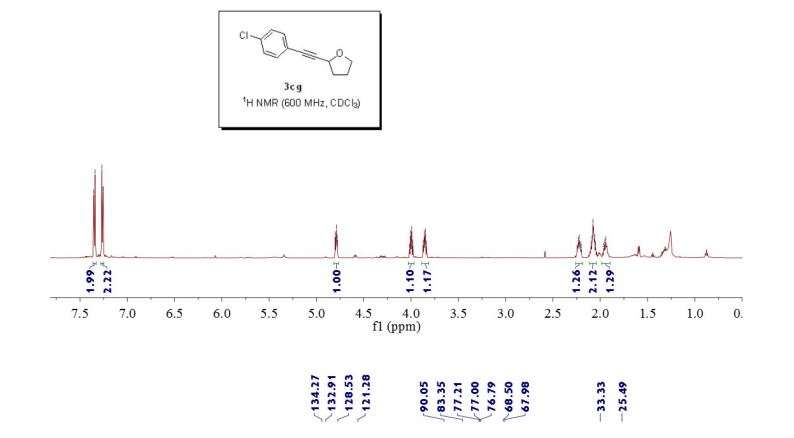
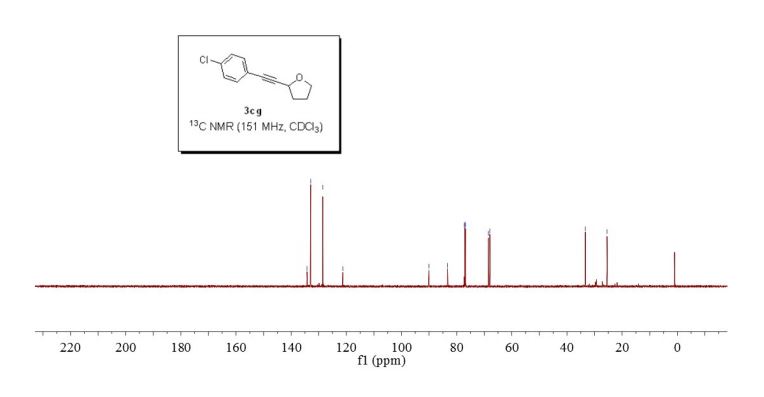
2-((4-chlorophenyl)ethynyl)tetrahydrofuran (3cg) ref 1 : Following general procedure, The product was purified by flash column chromatography on silica gel (petroleum ether) and 1c : 2g = 1:69, obtained in 70 % yield as a pale yellow oil (28.8 mg).
1H NMR (600 MHz, CDCl3) δ 7.35 (d, J = 8.4 Hz, 2H), 7.28 – 7.25 (m, 2H), 4.82 – 4.77 (m, 1H), 4.00 (dd, J = 14.6, 7.1 Hz, 1H), 3.85 (dd, J = 13.6, 7.8 Hz, 1H), 2.26 – 2.19 (m, 1H), 2.11 – 2.04 (m, 2H), 1.95 (dd, J = 13.3, 5.8 Hz, 1H).
Wan, M.; Meng, Z.; Lou, H.; Liu, L. Angew. Chem. Int. Ed. 2014, 126, 14065.
13C NMR (151 MHz, CDCl3) δ 134.2, 132.9, 128.5, 121.2, 90.0, 83.3, 68.5, 67.9, 33.3, 25.4.
//////////Directed alkynylation, unactivated C(sp3)-H bonds, ethynylbenziodoxolones, DTBP