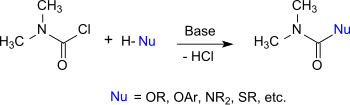|
|
| Identifiers | |
|---|---|
| 79-44-7 | |
| ECHA InfoCard | 100.001.099 |
| PubChem | 6598 |
| Properties | |
| C3H6ClNO | |
Dimethylcarbamoyl Chloride

Mechanisms for the Formation of Dimethylcarbamoyl Chloride
Thionyl chloride is the most common reagent in process chemistry for the conversion of a carboxylic acid to an acid chloride. One of the primary factors is cost, since the reagent is inexpensive and represents one of the most cost-efficient ways of preparing acid chlorides. However, one disadvantage of thionyl chloride is the potential formation of dimethylcarbamoyl chloride, a known carcinogen in animal models, when used in combination with DMF as catalyst.
Dimethylcarbamoyl chloride is a reagent for transferring a dimethylcarbonyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans,[1] dimethylcarbamoyl chloride can only be used under stringent safety precautions.
Production and occurrence
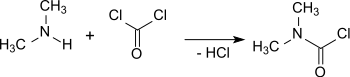
The production of dimethylcarbamoyl chloride from phosgene and dimethylamine (DMA) was reported as early as 1879 (reported as “Dimethylharnstoffchlorid” – dimethylurea chloride).[2]
Dimethylcarbamoyl chloride can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor.[3] To suppress the formation of ureas excessive phosgene is used (in a 3:1 ratio).
The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and a aqueous dimethylamine solution in the two-phase system benzene+xylene/water in a stirred reactor with sodium hydroxide as an acid scavenger. However, considerably lower yields (56%) are achieved due to the hydrolysis sensitivity of dimethylcarbamoyl chloride .[4]
Dimethylcarbamoyl chloride is also formed (together with methyl chloride) when reacting phosgene with trimethylamine.[5]
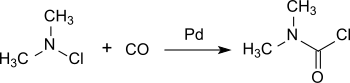
A more recent process is based on dimethylamine chloride, which is converted practically quantitatively to dimethylcarbamoyl chloride on a palladium catalyst under pressure with carbon monoxide at room temperature.[6]
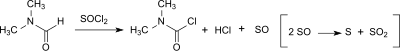
Dicarbamoyl chloride can also be formed in small amounts (0-20 ppm) from dimethylformamide (DMF) in the Vilsmeier-Haack reaction[7] or when DMF is used as a catalyst in the reaction of carboxylic acids with thionyl chloride to the corresponding carboxylic acid chlorides.[8]
The tendency towards dicarbamoyl chloride formation depends on the chlorination reagent (thionyl chloride> oxalyl chloride> phosphorus oxychloride) and is higher in the presence of a base. However, dicarbamoyl chloride hydrolyses very quickly to dimethylamine, hydrochloric acid and carbon dioxide (with a half-life of about 6 minutes at 0 °C) so that less than 3 ppm of dicarbamoyl chloride are found in the Vilsmeier product after aqueous work-up.[9]
Properties[edit]
Dimethylcarbamoyl chloride is a clear, colorless, corrosive and flammable liquid with a pungent odor and a tear-penetrating effect, which decomposes rapidly in water.[10]Because of its unpleasant, toxic, mutagenic and carcinogenic properties[11][12] it has to be used under extreme precautions.
Dimethylcarbamoyl chloride behaves like an acid chloride whose chlorine atom can be exchanged for other nucleophiles. Therefore, it reacts with alcohols, phenols and oximes to the corresponding N, N-dimethylcarbamates, with thiols to thiolourethanes, with amines and hydroxylamine to substituted ureas, and with imidazoles and triazoles to carbamoylazoles.[13]
Dimethylcarbamoyl chloride is less reactive and less selective to substrates with multiple nucleophilic centers than conventional acid chlorides.
Unsaturated conjugated aldehydes such as (2E)-butenal react with dimethylcarbamoyl chloride forming dienyl carbamates, which can be used as dienes in Diels-Alder reactions.[14]
Alkali metal carboxylates react with dimethylcarbamoyl chloride forming the corresponding dimethylamides. Dimethylcarbamoyl chloride reacts with anhydrous sodium carbonate[15] or with excess dimethylamine to tetramethylurea.[16]
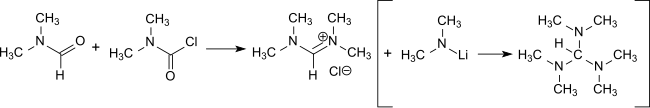
The reaction of dimethylcarbamoyl chloride with DMF forms tetramethylformamidinium[17] chloride which is a major intermediate in the preparation of tris(dimethylamino)methane, a reagent for the introduction of enamine functions in conjunction with activated methylene groups[18] and the preparation of amidines.[19]
Dimethylcarbamoyl chloride is a starting material for the insecticide class of the dimethyl carbamates[20] which act as inhibitors of acetylcholinesterase, including dimetilane,[21]and the related compounds isolane, pirimicarb and triazamate.
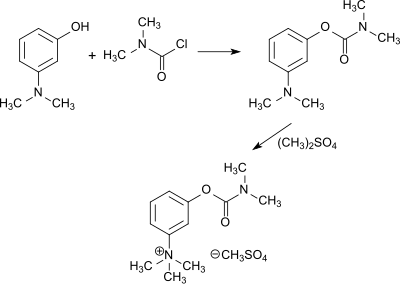
The quaternary ammonium compounds neostigmine[22] finds pharmaceutical applications as acetylcholinesterase inhibitors. It is obtained from 3-dimethylaminophenol and dimethylcarbamoyl chloride and subsequent quaternization with methyl bromide or dimethyl sulfate[23]
and pyridostigmine, which is obtainable from 3-hydroxypyridine and dimethylcarbamoyl chloride and subsequent reaction with methyl bromide.[24]
Dimethylcarbamoyl chloride is also used in the synthesis of the benzodiazepine camazepam.[25]
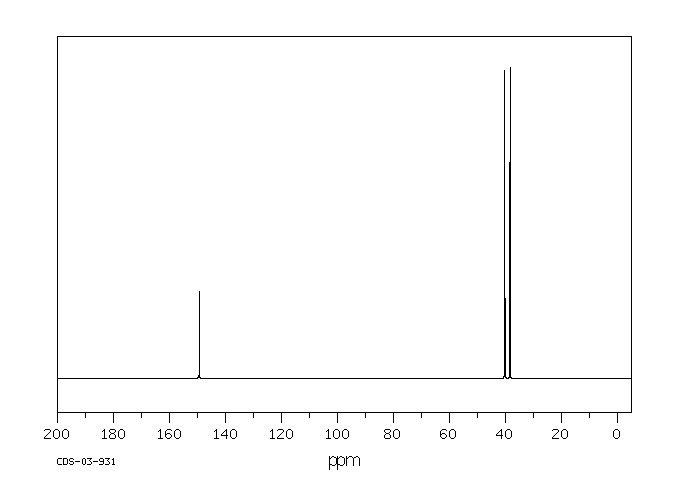
13c NMR
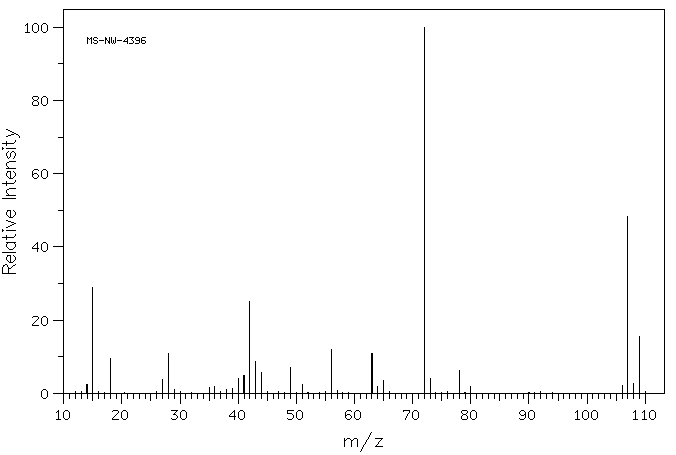
MASS
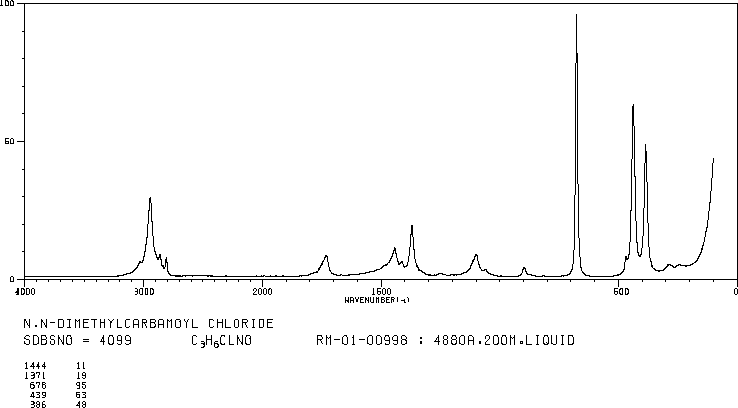
RAMAN
References
- Jump up^ R.P. Pohanish (2011) (in German), Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens, 6th Edition, Amsterdam: Elsevier, pp. 1045–1047, ISBN 978-1437778694
- Jump up^ W. Michler; C. Escherich (1879), “Ueber mehrfach substituirte Harnstoffe” (in German), Ber. Dtsch. Chem. Ges. 12 (1): pp. 1162–1164, doi:10.1002/cber.187901201303
- Jump up^ R.J. Slocombe; E.A. Hardy; J.H. Saunders; R.L. Jenkins (1950), “Phosgene derivatives. The preparation of isocyanates, carbamyl chlorides and cyanuric acid” (in German), J. Am. Chem. Soc. 72 (5): pp. 1888–1891, doi:10.1002/ja01161a009
- Jump up^ G. Karimipour; S. Kowkabi; A. Naghiha (2015), “New aminoporphyrins bearing urea derivative substituents: synthesis, characterization, antibacterial and antifungal activity” (in German), Braz. Arch. Biol. Technol. 58 (3), doi:10.1590/S1516-891320500024
- Jump up^ H. Babad; A.G. Zeiler (1973), “Chemistry of Phosgene” (in German), Chem. Rev. 73 (1): pp. 75–91, doi:10.1021/cr60281a005
- Jump up^ T. Saegusa; T. Tsuda; Y. Isegawa (1971), “Carbamoyl chloride formation from chloramine and carbon monoxide” (in German), J. Org. Chem. 36 (6): pp. 858–860, doi:10.1021/jo00805a033
- Jump up^ M. Stare; K. Laniewski; A. Westermark; M. Sjögren; W. Tian (2009), “Investigation on the formation and hydrolysis of N,N-dimethylcarbamoyl chloride (DMCC) in Vilsmeier reactions using /GC/MS as the analytical detection method” (in German), Org. Process Res. Dev. 13 (5): pp. 857–862, doi:10.1021/op900018f
- Jump up^ D. Levin (1997), “Potential toxicological concerns associated with carboxylic acid chlorination and other reactions” (in German), Org. Process Res. Dev. 1 (2): pp. 182, doi:10.1021/op970206t
- Jump up^ A. Queen (1967), “Kinetics of the hydrolysis of acyl chlorides in pure water” (in German), Canad. J. Chem. 45 (14): pp. 1619–1629, doi:10.1139/v67-264
- Jump up^ C.B. Kreutzberger; R.A. Olofson (2001), “Dimethylcarbamoyl Chloride” (in German), e-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rd319
- Jump up^ P. Jäger; C.N. Rentzea; H. Kieczka (2014) (in German), Carbamates and Carbamoyl Chloride, in Ullmann’s Fine Chemicals, Weinheim: Wiley-VCH, pp. 57–58, ISBN 978-3-527-33477-3
- Jump up^ “Dimethylcarbamoyl Chloride, CAS No. 79-44-7” (PDF). Report on Carcinogens, Thirteenth Edition (in German). National Toxicology Program, Department of Health and Human Services. Retrieved 2016-09-25.
- Jump up^ C.B. Kreutzberger, R.A. Olofson (2007-02-01). “Dimethylcarbamoyl Chloride” (in German). John Wiley&Sons, Ltd. Retrieved 2016-09-27.
- Jump up^ P.F. De Cusati; R.A. Olofson (1990), “A simple synthesis of 1-(1,3-butadienyl)carbonates and carbamates” (in German), Tetrahedron Lett. 31 (10): pp. 1405–1408, doi:10.1016/S0040-4039(00)88817-6
- Jump up^ J.K. Lawson Jr.; J.A.T. Croom (1963), “Dimethylamides from alkali carboxylates and dimethylcarbamoyl chloride” (in German), J. Org. Chem. 28 (1): pp. 232–235, doi:10.1021/jo1036a513
- Jump up^ US 3597478, M.L. Weakly, “Preparation of tetramethylurea”
- Jump up^ Z. Arnold (1959), “The preparation of tetramethylformamidinium salts and their vinylogues” (in German), Coll. Czech. Chem. Commun. 24: pp. 760–765, doi:10.1135/cccc19590760
- Jump up^ H. Meerwein; W. Florian; N. Schön; G. Stopp (1961), “Über Säureamidacetale, Harnstoffacetale und Lactamacetale” (in German), Justus Liebigs Ann. Chem. 641 (1): pp. 1–39, doi:10.1002/jlac.19616410102
- Jump up^ H. Bredereck; F. Effenberger; Th. Brendle (1966), “Synthese und Reaktionen von Trisdimethylaminomethan” (in German), Angew. Chem. 78 (2): pp. 147–148, doi:10.1002/ange.19660780212
- Jump up^ “Compendium of Pesticide Common Names” (in German). Alan Wood. Retrieved 2016-09-27.
- Jump up^ US 3452043, T. Grauer, H. Urwyler, “Production of 1-N,N-dimethylcarbamoyl-5-methyl-3-N,N-dimethyl-carbamoyl-oxy-pyrazole”
- Jump up^ J.A. Aeschlimann; M. Reinert (1931), “Pharmacological action of some analogues of physostigmine” (in German), J. Pharmacol. Exp. Ther. 43 (3): pp. 413–444
- Jump up^ US 1905990, J.A. Aeschlimann, “Disubstituted carbamic acid esters of phenols containing a basic constituent”
- Jump up^ US 2572579, “Disubstituted carbamic acid esters of 3-hydroxy-1-alkyl-pyridinium salts”
- Jump up^ DOS 2448015, “Verfahren zur Herstellung des 3-N,N-Dimethylcarbamoyl-oxy-1-methyl-5-phenyl-7-chlor-1,3-dihydro-2H-1,4-benzodiazepin-2-on”
/////////////