BERAPROST
https://www.ama-assn.org/resources/doc/usan/beraprost.pdf
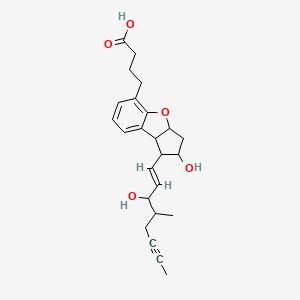
2,3,3a,8b-tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-1H-cyclopenta(b)benzofuran-5-butanoic acid
(±)-(IR*,2R*,3aS*,8bS*)-2,3,3a,8b-tetrahydro-2-hydroxy-1-[(E)-(3S*)-3-hydroxy-4-methyl-1-octene-6-inyl]-1H-cyclopenta[b]benzofuran-5-butyric acid
rac-4-{(1R,2R,3aS,8bS)-2-hydroxy-1-[(1E,3S,4RS)-3-hydroxy-4-methyloct-1-en-6-ynyl]-2,3,3a,8b-tetrahydro-1H-cyclopenta[b][1]benzofuran-5-yl}butanoic acid
- Beraprost
- Beraprostum
- Beraprostum [INN-Latin]
- MDL 201229
- MDL-201229
- ML 1229
- ML-1229
- UNII-35E3NJJ4O6
Beraprost is a synthetic analogue of prostacyclin, under clinical trials for the treatment of pulmonary hypertension. It is also being studied for use in avoiding reperfusion injury.
As an analogue of prostacyclin PGI2, beraprost effects vasodilation, which in turn lowers the blood pressure. Beraprost also inhibits plateletaggregation, though the role this phenomenon may play in relation to pulmonary hypertension has yet to be determined.
Beraprost …sodium salt
ML 1129; Procyclin; TRK 100 (CAS 88475-69-8)

| Synonyms |
|
|---|---|
| Formal Name | 2,3,3a,8b-tetrahydro-2-hydroxy-1-(3-hydroxy-4-methyl-1-octen-6-ynyl)-1H-cyclopenta[b]benzofuran-5-butanoic acid, monosodium salt |
| CAS Number | 88475-69-8 |
| Molecular Formula | C24H29O5 · Na |
| Formula Weight | 420.5 |
- Beraprost sodium is a prostacyclin analog and an NOS3 expression enhancer that was first launched in 1992 in Japan pursuant to a collaboration between Astellas Pharma and Toray for the oral treatment of peripheral vascular disease (PVD), including Raynaud’s syndrome and Buerger’s disease. In 2000, the drug was commercialized for the treatment of pulmonary hypertension. Development for the oral treatment of intermittent claudication associated with arteriosclerosis obliterans (ASO) was discontinued at Kaken and United Therapeutics after the product failed to demonstrate statistically significant results in a phase III efficacy trial.
- In terms of clinical development, beraprost sodium is currently in phase II clinical trials at Kaken for the treatment of lumbar spinal canal stenosis and at Astellas Pharma for the oral treatment of primary chronic renal failure. The company is also conducting phase III trials for the treatment of nephrosclerosis. The drug has also been studied through phase II clinical trials at Kaken for the oral treatment of diabetic neuropathy, but recent progress reports for this indication have not been made available.
- Beraprost is an oral form of prostacyclin, a member of the family of lipid molecules known as eicosanoids. Prostacyclin is produced in the endothelial cells from prostaglandin H2 by the action of the enzyme prostacyclin synthase. It has been shown to keep blood vessels dilated and free of platelet aggregation.
- Beraprost sodium was originally developed at Toray in Japan, and rights to the drug were subsequently acquired by Astellas Pharma. A 1972 alliance between Toray and Kaken Pharmaceutical to develop and commercialize prostaglandin led to a later collaboration agreement for the development of beraprost. In 1990, Toray granted the right to market the drug to Sanofi (formerly known as sanofi-aventis), a licensing agreement that was later expanded to include Canada, the U.S., South America, Africa, Southeast Asia, South Asia, Korea and China. In September 1996, Bristol-Myers Squibb entered into separate agreements with Sanofi and Toray to acquire all development and marketing rights to beraprost in the U.S. and Canada. In January 1999, United Therapeutics and Toray agreed to cooperatively test the drug in North America, and in July 2000, a new agreement was signed pursuant to which United Therapeutics gained exclusive North American rights to develop and commercialize sustained-release formulations of beraprost for all vascular and cardiovascular diseases. In 1999, orphan drug designation was received in the U.S. for the treatment of pulmonary arterial hypertension associated with any New York Heart Association classification (Class I, II, III, or IV). In 2011, orphan drug designation was assigned in the U.S. for the treatment of pulmonary arterial hypertension.
- The compound name of beraprost which is used as an antimetastasis agent of malignant tumors according to the present invention is (±)-(IR*,2R*,3aS*,8bS*)-2,3,3a,8b-tetrahydro-2-hydroxy-1-[(E)-(3S*)-3-hydroxy-4-methyl-1-octene-6-inyl]-1H-cyclopenta[b]benzofuran-5-butyric acid. This compound has the following structure.
Beraprost is described in Japanese Laid-open Patent Application (Kokai) Nos. 58-32277, 57-144276 and 58-124778 and the like as a PGI₂ derivative having a structure in which the exoenol moiety characteristic to beraprost is converted to inter-m-phenylene structure. However, it is not known that beraprost has an activity to inhibit metastasis of malignant tumors.
- The beraprost which is an effective ingredient of the agent of the present invention includes not only racemic body, but also d-body and l-body. Beraprost can be produced by, for example, the method described in the above-mentioned Japanese Laid-open Patent Application (Kokai) No. 58-124778. The salts of beraprost include any pharmaceutically acceptable salts including alkaline metal salts such as sodium salt and potassium salt; alkaline earth metal salts such as magnesium salt and calcium salt; ammonium salt; primary, secondary and tertiary amine salts; and basic amino acid salts.
…………………..
EXAMPLE 6 Beraprost of the Formula (I)
0.246 g (0.6 mmol) of compound of the general formula (II) obtained in Example 5 is dissolved in 1 ml of methanol and 1 ml of 1 M aqueous sodium hydroxide solution is added dropwise slowly thereto. After stirring for an hour the methanol is distilled off from the reaction mixture in vacuum. The aqueous residue is diluted with 10 ml of water extracted with methyl-tert.butyl-ether and the combined organic phase is washed with saturated NaCl solution, dried on Na2SO4 and evaporated. The residue of evaporation is crystallized from ethylacetate-hexane mixture and the pure above mentioned title compound is obtained as colourless crystals.
Yield: 0.21 g (87%)
TLC-Rf (toluene-dioxan-acetic acid 20:10:1)=0.41
Melting point: 98–112° C.
1H NMR (400 MHz, CDCl3), δH (ppm): 1.00d, 1.03d [3H; J=6.8 Hz; 21-H3]; 1.79m [1H; 16-H]; 1.80t, 1.81t [3H, J=2.5,2.4 Hz; 20-H3]; 2.3–1.9m [5H, 3-H2, 10Hb, 17-H2]; 2.34t [1H; J=7.4 Hz; 2-H2]; 2.43m [1H; 12-H]; 2.64m [3H; 10-Ha, 4-H2]; 3.43t, 3.44t [1H, J=8.7,8.5 Hz; 8-H]; 3.92m [1H; 11-H]; 4.07t, 4.17t [1H, J=7.3,5.6 Hz; 15-H]; 4.3b [2H; OH]; 5.09m [1H, 9-H]; 5.58dd, 5.61dd [1H; J=15.3,6.5 Hz; 14-H]; 5.67dd, 5.68dd [1H; J=15.3,8.0 Hz; 13-H]; 6.77m [1H; 2′-H]; 6.95m [2H; 1′-H,3′-H]13C NMR (100 MHz, CDCl3), δC (ppm): 3.5, 3.6 [C-20]; 14.7, 15.8 [C-21]; 22.3, 22.6 [C-17]; 24.6 [C-2]; 29.1 [C-4]; 33.1 [C-3]; 38.2, 38.3 [C-16]; 41.2 [C-10]; 50.4 [C-8]; 58.8 [C-12]; 75.8, 76.3, 76.4 [C-11, C-15]; 77.2, 77.4 [C-18, C-19]; 84.5, 84.6 [C-9]; 120.6 [C-2′]; 121.9 [C-3′]; 123.2 [C-5]; 129.0 [C-1′]; 129.7 [C-7]; 132.3, 133.0, 133.8, 134.0 [C-13, C-14]; 157.2 [C-6]; 178.3 [C-1].
EXAMPLE 7 Beraprost Sodium Salt (The Sodium Salt of the Compound of Formula (I)
0.199 g of beraprost is dissolved in 2 ml of methanol, 0.5 ml of 1 M aqueous solution of sodium hydroxide is added thereto and after their mixing the solvent is evaporated in vacuum and thus the above title salt is obtained as colourless crystals.
Yield: 0.21 g (100%)
Melting point: >205° C.
1H NMR (400 MHz, DMSO-d6), δH (ppm): 0.90d, 0.92d [3H; J=6.7 Hz; 21-H3]; 1.75–1.55m [7H; 10Hb, 16-H, 3-H2, 20-H3]; 1.89t [2H, J=7.6 Hz; 2-H2]; 1.94m [1H; 17-Hb]; 2.16q [1H, J=8.5 Hz; 12-H]; 2.25m [1H; 17-Ha]; 2.44t [2H; J=7.5 Hz; 4-H2]; 2.50o [1H; 10-Ha]; 3.39t [1H, J=8.5 Hz; 8-H]; 3.72td [1H; J=8.5,6.1 Hz; 11-H]; 3.84t 3.96t [1H, J=6.5,6.0 Hz; 15-H]; 4.85b [2H, OH]; 5.01dt [1H, J=8.5,6.6 Hz; 9-H]; 5.46dd, 5.47dd [1H; J=15.4,6.5 Hz, J=15.4,6.0 Hz; 14-H]; 5.65dd, 5.66dd [1H; J=15.4,8.5 Hz; 13-H]; 6.71m [1H; 2′-H]; 6.92m [2H; 1′-H, 3′-H] During the above thin layer chromatography (TLC) procedures we used plates MERCK Kieselgel 60 F254, thickness of layer is 0.2 mm, length of plates is 5 cm.
…………….
- Reaction Scheme A.
-
The starting material of bromocarboxylic acid, Compound 1, and the process for the preparation thereof are disclosed in Japanese Patent Application No. 29637/81.
- Scheme B.
REACTION SCHEME B
-
- REACTION SCHEME C
-
Org Lett 2012, 14(1): 299

EP0024943A1 Sep 2, 1980 Mar 11, 1981 Toray Industries, Inc. 5,6,7-Trinor-4,8-inter-m-phenylene PGI2 derivatives and pharmaceutical compositions containing them EP0084856A1 Jan 19, 1983 Aug 3, 1983 Toray Industries, Inc. 5,6,7-Trinor-4, 8-inter-m-phenylene prostaglandin I2 derivatives JP3069909B Title not available
-