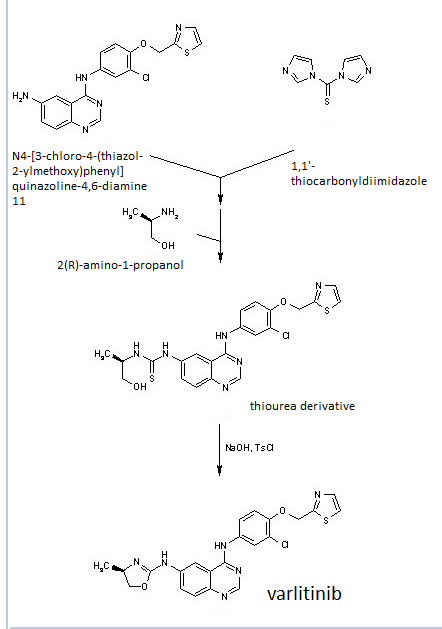
(R)-N4-[3-Chloro-4-(thiazol-2-ylmethoxy)-phenyl]-N6-(4-methyl-4,5-dihydro-oxazol-2-yl)-quinazoline-4,6-diamine
.gif)
ASLAN001 , Varlitinib
C22H19ClN6O2S
Molecular Weight: 466.94
Elemental Analysis: C, 56.59; H, 4.10; Cl, 7.59; N, 18.00; O, 6.85; S, 6.87
CAS: 845272-21-1 (Varlitinib); 1146629-86-8 (Varlitinib tosylate).
ASLAN001; ASLAN-001; ASLAN 001; AR 00334543; ARRY-334543; ARRY334543; ARRY-543; ARRY543; ARRY 543.
(R)-N4-(3-chloro-4-(thiazol-2-ylmethoxy)phenyl)-N6-(4-methyl-4,5-dihydrooxazol-2-yl)quinazoline-4,6-diamine.
(R)-4-[[3-Chloro-4-[(thiazol-2-yl)methoxy]phenyl]amino]-6-[(4-methyl-4,5-dihydrooxazol-2-yl)amino]quinazoline
4,6-Quinazolinediamine, N4-[3-chloro-4-(2-thiazolylmethoxy)phenyl]-N6-[(4R)-4,5-dihydro-4-methyl-2-oxazolyl]-
ASLAN Pharmaceuticals, a Singapore-based drugmaker, announced The Food and Drug Administration (FDA) gave an orphan drug designation on August 13 to its pan-HER inhibitor ASLAN001 (varlitinib), a drug candidate created to treat a destructive form of bile duct cancer called cholangiocarcinoma that has no known cure. ………http://www.dddmag.com/news/2015/08/aslan-pharmaceuticals-gains-orphan-designation-rare-cancer-drug
Current developer: Array Biopharma Inc,

Varlitinib, also known as ARRY-543 and ASLAN001, is an orally bioavailable inhibitor of the epidermal growth factor receptor family with potential antineoplastic activity.
Varlitinib (ASLAN-001) is an oncolytic drug in phase II clinical trials at ASLAN Pharmaceuticals for the treatment of gastric cancer and for the treatment of metastatic breast cancer in combination with capecitabine. Clinical development is also ongoing for the treatment of solid tumors in combination with cisplatin/FU and cisplatin/capecitabine. The product had been in phase I/II clinical trials at Array BioPharma for the treatment of patients with advanced pancreatic cancer. Phase II clinical trials had also been ongoing for the treatment of solid tumors. No recent development has been reported for this research
Varlitinib selectively and reversibly binds to both EGFR (ErbB-1) and Her-2/neu (ErbB-2) and prevents their phosphorylation and activation, which may result in inhibition of the associated signal transduction pathways, inhibition of cellular proliferation and cell death. EGFR and Her-2 play important roles in cell proliferation and differentiation and are upregulated in various human tumor cell types. Due to the dual inhibition of both EGFR and Her-2, this agent may be therapeutically more effective than agents that inhibit EGFR or Her-2 alone.
The drug is a dual inhibitor of the ErB-2 and EGFR receptor kinases, both of which have been shown to stimulate aberrant growth, prolong survival and promote differentiation of many tumor types. The compound behaves as a reversible ATP-competitive inhibitor with nanomolar potency both in vitro and in cell-based proliferation assays.
In 2011, the compound was licensed to Aslan Pharmaceuticals by Array BioPharma worldwide for the treatment of solid tumors, initially targeting patients with gastric cancer through a development program conducted in Asia.
In 2015, orphan drug designation was assigned to the compound in the U.S. for the treatment of cholangiocarcinoma.
SEE NMR ………….http://www.medkoo.com/Product-Data/Varlitinib/Varlitinib-QC-KB20121128web.pdf
……………..
https://www.google.co.in/patents/US20050043334
Example 52
(R)-N4-[3-Chloro-4-(thiazol-2-ylmethoxy)-phenyl]-N6-(4-methyl-4,5-dihydro-oxazol-2-yl)-quinazoline-4,6-diamine
Prepared using (R)-2-aminopropan-1-o1. MS APCI (+) m/z 467, 469 (M+1, Cl pattern) detected; 1H NMR (400 mHz, DMSO-D6) δ 9.53 (s, 1H), 8.47 (s, 1H), 8.09 (s, 1H), 7.86 (d, 1H), 7.81 (d, 1H), 7.77 (d, 1H), 7.69 (m, 3H), 7.32 (d, 1H), 7.02 (s, 1H), 5.54 (s, 2H), 4.47 (m, 1H), 3.99 (m, 1H), 3.90 (m, 1H), 1.18 (d, 3H).
Example 53
(S)-N4-[3-Chloro-4-(thiazol-2-ylmethoxy)-phenyl]-N6-(4-methyl-4,5-dihydro-oxazol-2-yl)-quinazoline-4,6-diamine
Prepared using (S)-2-amino-propan-1-o1. MS APCI (+) m/z 467, 469 (M+1, Cl pattern) detected; 1H NMR (400 mHz, DMSO-D6) δ 9.53 (s, 1H), 8.47 (s, 1H), 8.09 (s, 1H), 7.86 (d, 1H), 7.81 (d, 1H), 7.77 (d, 1H), 7.69 (m, 3H), 7.32 (d, 1H), 7.02 (s, 1H), 5.54 (s, 2H), 4.47 (m, 1H), 3.99 (m, 1H), 3.90 (m, 1H), 1.18 (d, 3H).
………………
PATENT
http://www.google.co.in/patents/WO2005016346A1?cl=en
Example 52
R VN4-r3-Chloro-4-(‘thiazol-2-v-metho-xy)-phenyll-N6-(4-methyl-4,5-dihvdro-oxazol- 2-yl)-quinazoUne-4,6-diamine
[00194] Prepared using (R)-2-aminopropan- 1 -ol. MS APCI (+) m/z 467, 469
(M+l, CI pattern) detected; 1H NMR (400 mHz, DMSO-D6) δ 9.53 (s, IH), 8.47 (s, IH), 8.09 (s, IH), 7.86 (d, IH), 7.81 (d, IH), 7.77 (d, IH), 7.69 (m, 3H), 7.32 (d, IH), 7.02 (s, IH), 5.54 (s, 2H), 4.47 (m, IH), 3.99 (m, IH), 3.90 (m, IH), 1.18 (d, 3H). Example 53
(S)-N4-|“3-Chloro-4- thiazol-2-ylmethoxy)-phenyll-N6-(‘4-methyl-4,5-dihvdro-oxazol- 2-yl)-quinazoline-4,6-diamine [00195] Prepared using (S)-2-amino-propan- 1 -ol. MS APCI (+) m z 467, 469
(M+l, CI pattern) detected; 1H NMR (400 mHz, DMSO-D6) δ 9.53 (s, IH), 8.47 (s, IH), 8.09 (s, IH), 7.86 (d, IH), 7.81 (d, IH), 7.77 (d, IH), 7.69 (m, 3H), 7.32 (d, IH), 7.02 (s, IH), 5.54 (s, 2H), 4.47 (m, IH), 3.99 (m, IH), 3.90 (m, IH), 1.18 (d, 3H).
………
CAUTION a very similar molecule but not same
 NOTE……..METHYL NEXT TO OXYGEN ATOM
NOTE……..METHYL NEXT TO OXYGEN ATOM
Design, Synthesis and Bioactivities Evaluation of Novel Quinazoline Analogs Containing Oxazole Units
DOI: 10.1002/cjoc.201400271,…………http://onlinelibrary.wiley.com/doi/10.1002/cjoc.201400271/abstract;jsessionid=04211D7526D97240F44E4355C6C57F50.f01t01
CAUTION …THIS IS NOT SAME
A novel type of quinazoline derivatives, which were designed by the combination of quinazoline as the backbone and oxazole scaffold as the substituent, have been synthesized and their biological activities were evaluated for anti-proliferative activities and EGFR inhibitory potency. Compound 12b demonstrated the most potent inhibitory activity (IC50=0.95 µmol/L for EGFR), which could be optimized as a potential EGFR inhibitor in the further study. The structures of the synthesized quinazoline analogs and all intermediates were comfirmed by 1H and 13C NMR, 2D NMR spectra, IR spectra and MS spectra.
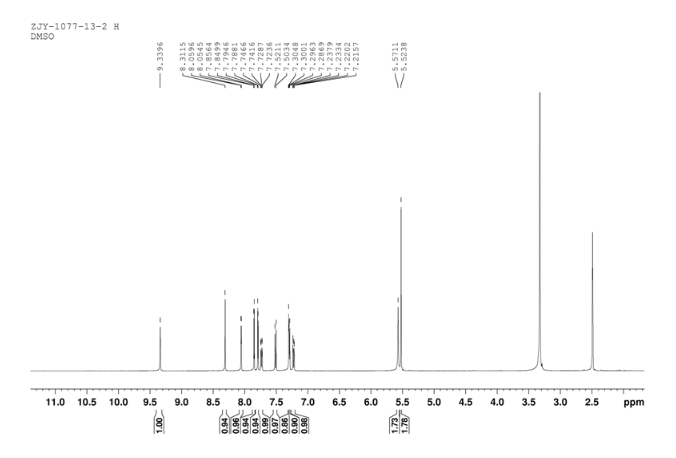
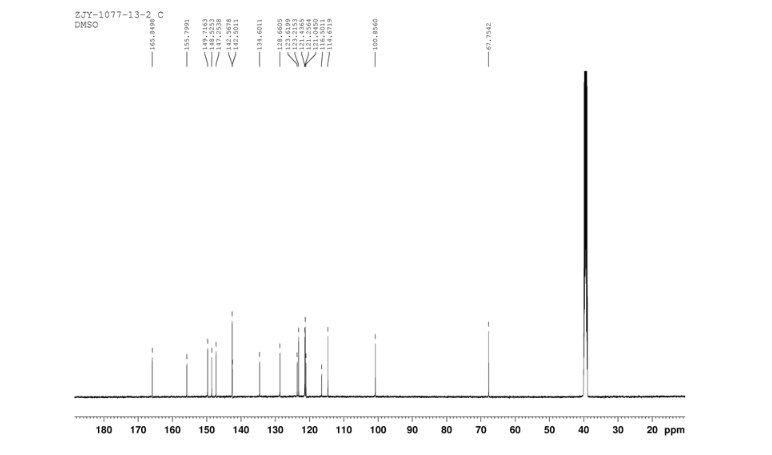
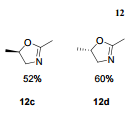
12c: Employing the same method as above, compound 12c was prepared and the amino alcohol was (S)-2-amino-propan-1-ol. Yellow solid, yield 52 %. m.p. 243-244 °C; [α] 20D =﹢22.5 ° (c 1.0, CH3CN); 1 H NMR (DMSO-D6): δ 9.54 (s, 1 H), 8.46 (s, 1 H), 8.06 (s, 2 H), 7.85 (d, 2 H, J=3.3 Hz), 7.79 (d, 2 H, J=3.3 Hz), 7.75 (d, 1 H, J=8.9 Hz), 7.64 (d, 1 H, J=8.3 Hz), 7.30 (d, 1 H, J=9.0 Hz), 5.54 (s, 2 H), 4.76 (m, 1 H), 3.72 (s, 1 H), 3.19 (s, 1 H), 1.34 (d, 3 H, J=6.15 Hz). 13C NMR (DMSO-D6) δ: 165.8, 156.9, 152.0, 148.8, 145.3, 142.6, 134.3, 128.7, 128.0, 123.5, 121.7, 121.3, 121.0, 115.6, 114.6, 72.5, 67.7, 63.0, 29.8, 29.0, 20.0, 13.9. IR (KBr) ν: 3439, 3278, 3101, 2925, 1660, 1631, 1601, 1557, 1500, 1428, 1404, 1384, 1329, 1291, 1257, 1225, 1052 cm-1. Anal. calcd for C22H19N6O2SCl: C 55.59, H 4.10, N 18.00, O 6.85; found C 55.55, H 4.13, N 18.02, O 6.78; MS (ESI) m/z: 467.2 (M+H).
12d: Employing the same method as above, compound 12d was prepared and the amino alcohol was (R)-2-amino-propan-1-ol. Yellow solid, yield 60%. m.p. 242-243 °C; [α] 20D = ﹣22.3 ° (c 1.0, CH3CN); 1 H NMR (DMSO-D6): δ 9.52 (s, 1 H), 8.80 (s, 1 H), 8.52 (dd, 1 H, J=2.7 Hz, J=8.9 Hz), 8.45 (s, 1 H), 8.30 (s, 1 H), 8.07 (s, 1 H), 7.85 (d, 1 H, J=3.2 Hz), 7.79 (d, 1 H, J=3.2 Hz), 7.75 (s, 1 H), 7.63 (d, 1 H, J=8.2 Hz), 7.31 (d, 1 H, J=9.0 Hz), 5.53 (s, 2 H), 4.76 (m, 1 H), 3.81 (s, 1 H), 3.19 (s, 1 H), 1.34 (d, 3 H, J=6.2 Hz). 13C NMR (DMSO-D6) δ: 165.8, 156.9, 152.0, 148.8, 145.3, 142.6, 134.3, 128.7, 128.0, 123.5, 121.7, 121.3, 121.0, 115.6, 114.6, 72.5, 67.7, 63.0, 29.8, 29.0, 20.0, 13.9. IR (KBr) ν: 3439, 3278, 3101, 2925, 1660, 1631, 1601, 1557, 1500, 1428, 1404, 1384, 1329, 1291, 1257, 1225, 1052 cm-1. Anal. calcd for C22H19N6O2SCl: C 55.59, H 4.10, N 18.00, O 6.85; found C 55.55, H 4.13, N 18.02, O 6.78; MS (ESI) m/z: 467.20 (M+H).
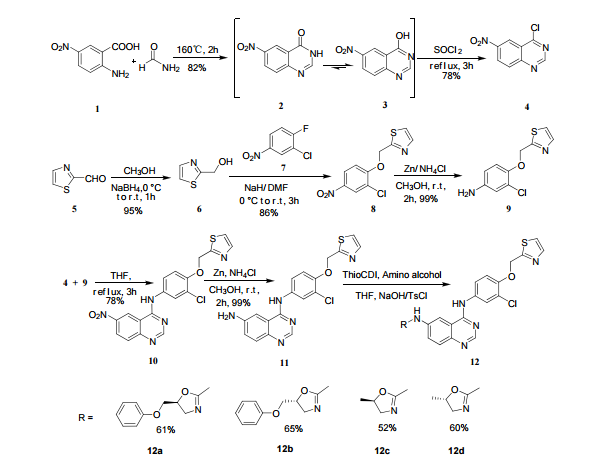
The above paper allows you to synthesize the key amino int 11 ………N4-(3-chloro-4-(thiazol-2-ylmethoxy)phenyl)quinazoline-4,6-diamine (11)
this can be applied to varlitinib till int 11
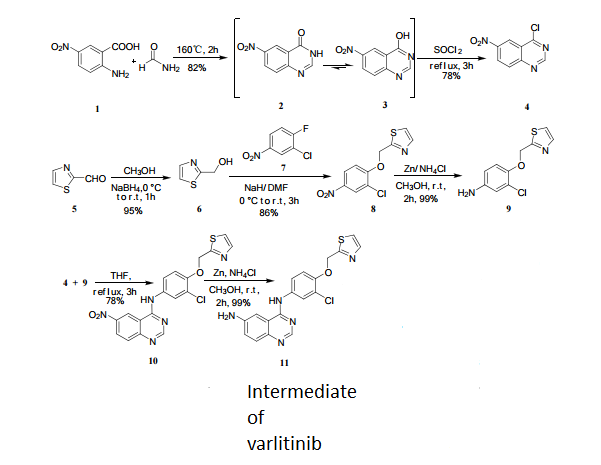
6-Nitro-4-hydroxyquinazoline (3)
2-amino-5-nitrobenzoic acid (5.46 g, 30 mmol) was added to a 250 mL flask equipped with a reflux condenser. Then 50 mL formamide was added. The mixture was heated with vigorous stirring at 160 °C for 3 h. After cooling the solution was poured in ice-water to give 3 in almost pure form (Yellow solid 4.70 g, yield 82.0%). m.p. 317-318 °C; 1 H NMR (DMSO-d6): δ 12.74 (1 H, s, OH, exchangeable), 8.78 (1 H, d, J=2.4 Hz), 8.53 (1 H, dd, J=2.6 Hz, 9.0 Hz), 8.30 (s, 1 H), 7.84 (1 H, d, J=9.0 Hz); 13C NMR (DMSO-d6) δ: 160.1, 152.9, 148.9, 145.0, 129.1, 128.3, 122.7, 121.9. IR (KBr) ν: 3172, 3046, 2879, 1674, 1615, 1577, 1514, 1491, 1469, 1343, 1289, 1242, 1167, 1112, 928, 920, 901, 803, 753, 630, 574, 531 cm-1. Anal. calcd for C8H5N3O3: C 50.27, H 2.64, N 21.98; found C 50.30, H 2.65, N 21.96; MS (ESI) m/z: 189.97 (M-H).
IR
4-chloro-6-Nitroquinazoline (4)
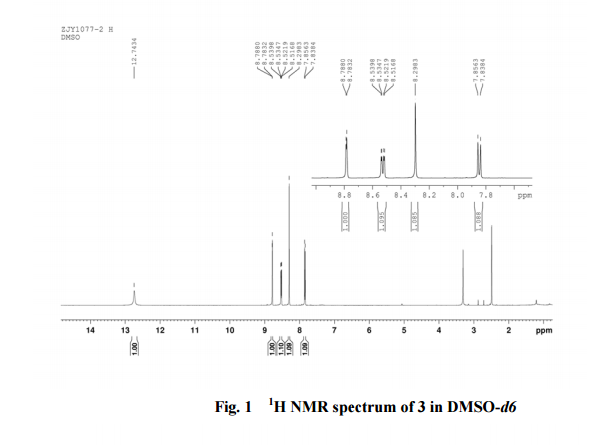
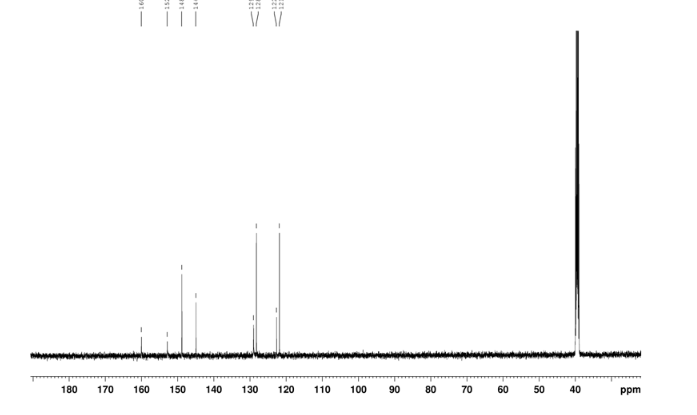
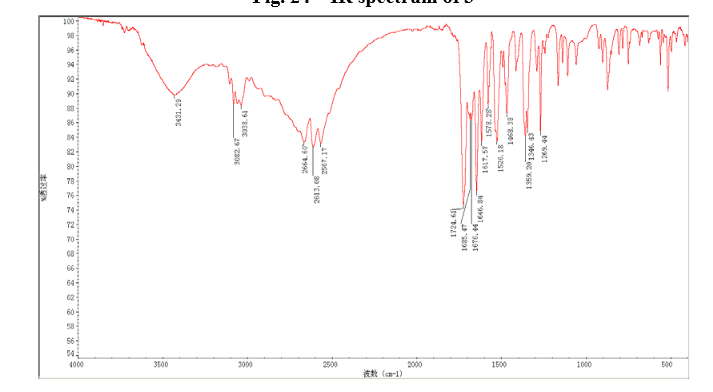
In a 100 mL flask equipped with a reflux condenser, 6-nitroquinazolin-4-one (2.86 g, 15 mmol) and thionyl chloride (SOCl2) 25 mL were added. The mixture was heated under reflux with vigorous stirring for 2 h. After the solution was clear, the reaction mixture was heated for another 2 h. Then, 150 mL of ice MeOH was dropped into it carefully, the mixture was extracted with CH2Cl2. The organic layer was S3 dried under MgSO4, filtered and the solvent removed to give 4-chloro-6-nitroquinazoline (4). Yellow solid 2.45 g, yield 78%. m.p. 134-135 °C; 1 H NMR (DMSO-d6): δ 8.80 (1 H, d, J=3.0 Hz), 8.54(1 H, dd, J=2.7 Hz, 9.0 Hz), 8.35(s, 1 H), 7.87 (1 H, d, J= 9.0 Hz); 13C NMR (DMSO-d6) δ: 160.0, 152.5, 149.1, 145.1, 128.7, 128.4, 122.7, 122.0. IR (KBr) ν: 3431, 3082, 3038, 2664, 2613, 2567, 1724, 1685, 1676, 1646, 1617, 1578, 1526, 1468, 1359, 1346, 1269 cm-1. Anal. calcd for C8H4N3O2Cl: C 45.84, H 1.92, N 20.05, O 15.27; found C 45.81, H 1.97, N 20.02, O 15.21; MS (ESI) m/z: 207.96 (M-H).
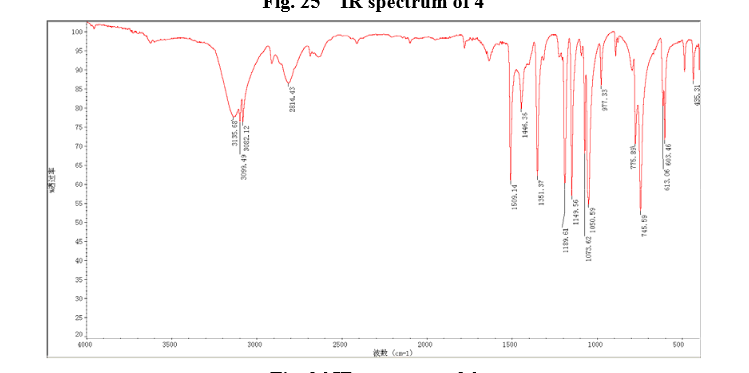
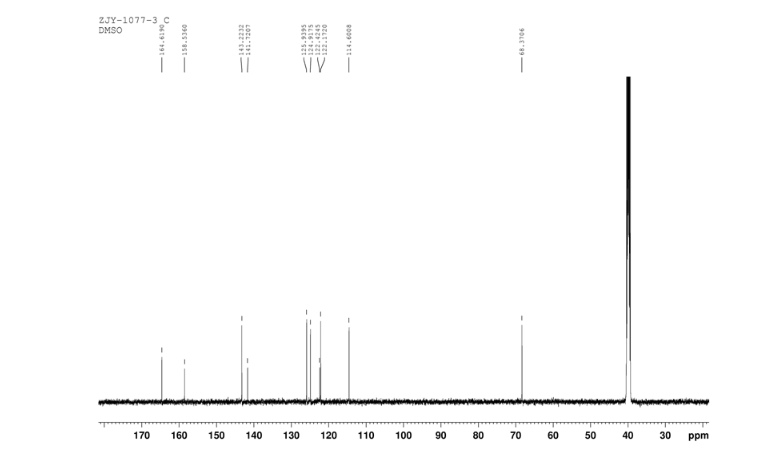
13C NMR OF4 IN DMSOD6
IR
Thiazol-2-yl-methano1 (6)
Sodium borohydride (16.0 g, 140 mmol) was added to a stirred solution of thiazole-2-carbaldehyde (24.2 g, 214 mmol) in MeOH (400 mL) at 0 °C . The reaction mixture was warmed to room temperature. After 1 hour, the reaction mixture was quenched by the addition of water and the organics were removed by concentration. The resulting aqueous mixture was extracted with EtOAc. The combined organic extracts were dried under Na2SO4 and concentrated to give thiazol-2-yl-methano1 (23.39 g, 95%). bp:75-76 °C (0.2 mmHg) [lit.[19] bp:70-80 °C (0.2 mmHg)]; m. p. 63-64 °C. 1 H NMR (CDCl3) δ 4.91 (s, 2 H), 5.1(br, l H), 7.28(d, 1 H, J=3.2 Hz), 7.68 (d, 1 H, J=2.9 Hz). IR (KBr) ν: 3135, 3099, 3082, 2814, 1509, 1446, 1351, 1189, 1149, 1073, 1050, 977, 775, 745, 613, 603 cm-1. Anal. calcd for C4H5NOS: C 41.72, H 4.38, N 12.16; found C 41.74, H 4.33, N 12.18; MS (ESI) m/z: 116.11 (M+H).
2-((2-Chloro-4-nitrophenoxy)methyl)thiazole (8)
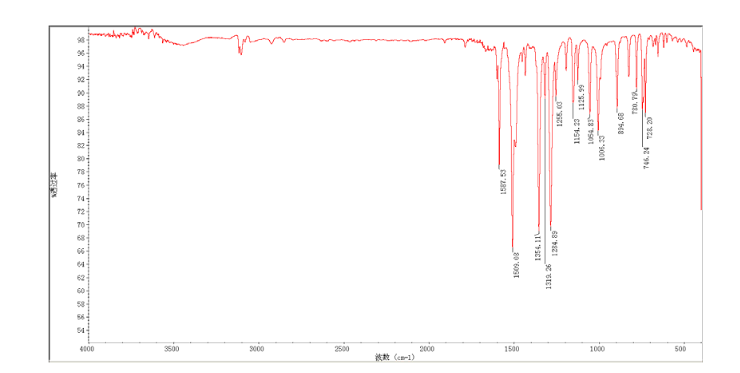
2-(2-chloro-4-nitro-phenoxymethy1)-thiazole was prepared by adding thiazol-2-yl-methanol (5.48 g, 47.65 mmol) to a slurry of sodium hydride (2.42 g of a 60% dispersion in oil, 60.5 mmol) in THF (50 ml) at 0 °C After several minutes, 2-chloro-1-fluoro- 4-nitro-benzene (7.58 g, 43.60 mmol) was added and the reaction mixture warmed to room temperature. The reaction mixture was stirred at room temperature for 3 h, and 60 °C for 16 h. After cooling to room temperature, the reaction mixture was poured into 300 mL water. The resulting precipitate was collected by filtration, washed with water, and dried in vacuo to give 2-(2- chloro-4-nitrophenoxymethy1)-thiazole (11.06 g, 86%) which was used in next step without further purification. m.p. 170-171 °C; 1 H NMR (DMSO-d6): δ 8.35 (1 H, d, J=2.8 Hz), 8.25 (1 H, dd, J=2.8 Hz, 9.15 Hz), 7.87 (1 H, d, J=3.3 Hz), 7.83(1 H, d, J=3.3 Hz), 7.54 (1 H, d, J=9.2 Hz), 5.73(s, 1 H); 13C NMR (DMSO-d6) δ: 164.2, 158.5, 143.2, 141.7, 125.9, 124.9, 122.4, 122.2, 114.6, 68.4; IR (KBr) ν: 3112, 3009, 1587, 1509, 1500, 1354, 1319, 1284, 1255, 1154, 1125, 1054, 1006, 894, 780, 746, 728 cm-1. Anal. calcd for C10H7N2O3SCl: C 44.37, H 2.61, N 10.35, O 17.73; found C 44.31, H 2.67, N 10.29; MS (ESI) m/z: 268.89 (M-H).
13C NMR OF 8 IN DMSOD6
3-Chloro-4-(thiazol-2-ylmethoxy)aniline (9)
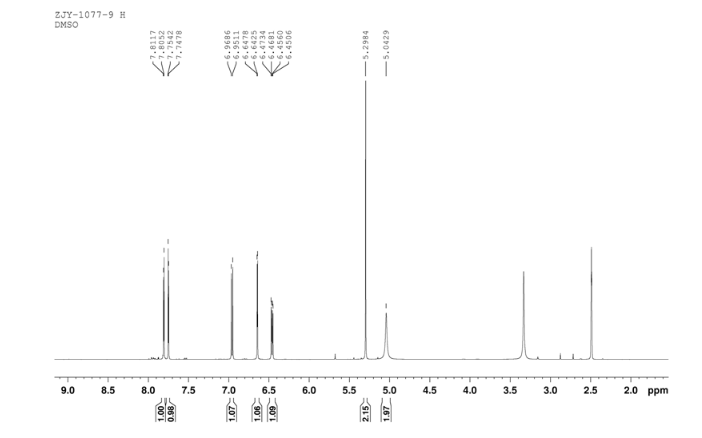
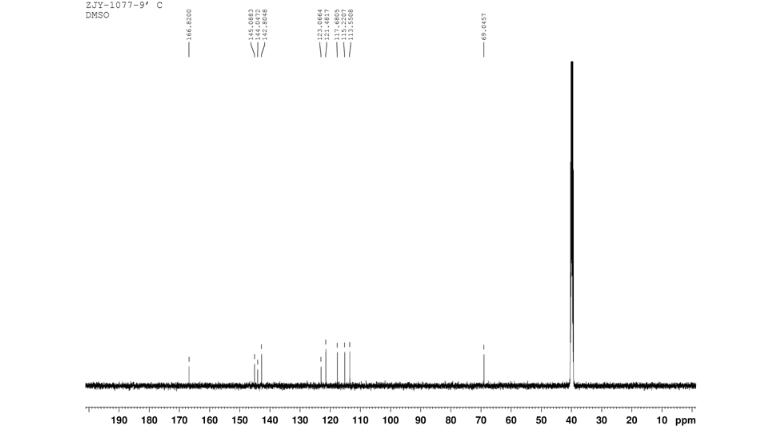
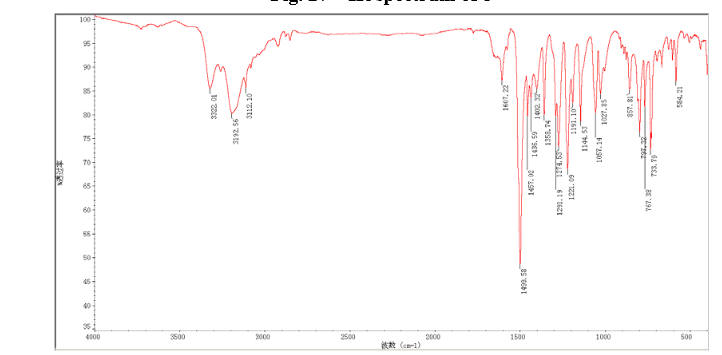
In a flask equipped with a reflux condenser, the compound 8 15.00 g (55.6 mmol), reduced zinc powder 14.44 g (222.0 mmo1, 4 eq), saturated ammonia chloride (5 mL) and methanol (100 mL) were mixed. The mixture was stirred at a temperature of 40 °C for 1.5 h. Then the zinc powder was filtered off, the filtrate was concentrated to obtain yellow solid 13.21 g, yield 99%. m.p. 60-61 °C; 1 H NMR (DMSO-d6): δ 7.80 (1 H, d, J=3.3 Hz), 7.75 (1 H, d, J=3.3 Hz), 6.96 (1 H, d, J=8.8 Hz), 6.64(1 H, d, J=2.7 Hz), 6.46 (1 H, dd, J=2.7 Hz, J=8.7 Hz), 5.30 (s, 2 H), 5.04 (s, 2 H, NH2, exchangeable); 13C NMR (DMSO-d6) δ: 166.8, 145.1, 144.1, 142.80, 123.1, 121.5, 117.7, 115.2, 113.6, 69.1. IR (KBr) ν: 3322, 3192, 3112, 1607, 1499, 1457, 1436, 1291, 1274, 1221, 1191, 1144, 1057, 1027, 857, 797, 767, 733, 584 cm-1. Anal. calcd for C10H9N2OSCl: C 49.90, H 3.77, N 11.64, O 6.65; found C 49.95, H 3.76, N 11.66, O 6.60; MS (ESI) m/z: 239.01 (M-H).
N-(3-chloro-4-(thiazol-2-ylmethoxy)phenyl)-6-nitro- quinazolin-4-amine(10)
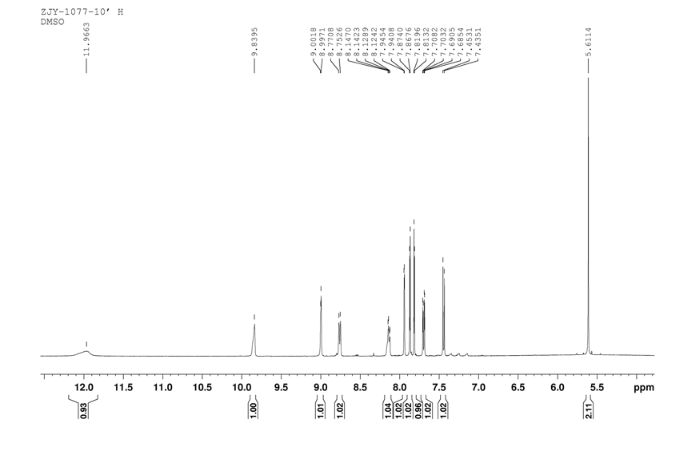
In a flask equipped with a reflux condenser, 6-nitro-4-chloro- quinazoline 8.0 g (38.3 mmol) and 3-Chloro-4-(thiazol-2-ylmethoxy)aniline 8.9 g (37.0 mmol) were dissolved into 150 mL of THF, and the solution was refluxed for 3 h.Then a lot of yellow solid was deposited. Then it was filtered affording to yellow solid 12.8 g, yield 81%. m.p. 183-184 °C (decompose); 1 H NMR (DMSO-d6): δ 11.97(s, 1 H, exchangeable), 9.84 (s, 1 H), 9.00 (s, 1 H), 8.76 (1 H, d, J=9.1 Hz), 8.12-8.14 (m, 1 H), 7.94 (1 H, d, J=2.3 Hz), 7.87 (1 H, d, J=3.2 Hz), 7.81 (1 H, d, J=3.2 Hz), 7.44 (1 H, d, J=9.0 Hz), 7.69 (1 H, dd, J=2.5 Hz, J=8.9 Hz), 5.61 (s, 2 H); 13C NMR (DMSO-d6) δ: 166.8, 145.1, 144.1, 142.8, 123.1, 121.5, 117.7, 115.2, 113.7, 69.1. IR (KBr) ν: 3442, 3100, 1636, 1618, 1570, 1552, 1523, 1492, 1442, 1400, 1377, 1344, 1301, 1267, 1069, 805 cm-1. Anal. calcd for C18H12N5O3SCl: C 52.24, H 2.92, N 16.92, O 11.60; found C 52.26, H 2.93, N 16.96, O 11.58; MS (ESI) m/z: 412.84 (M-H).
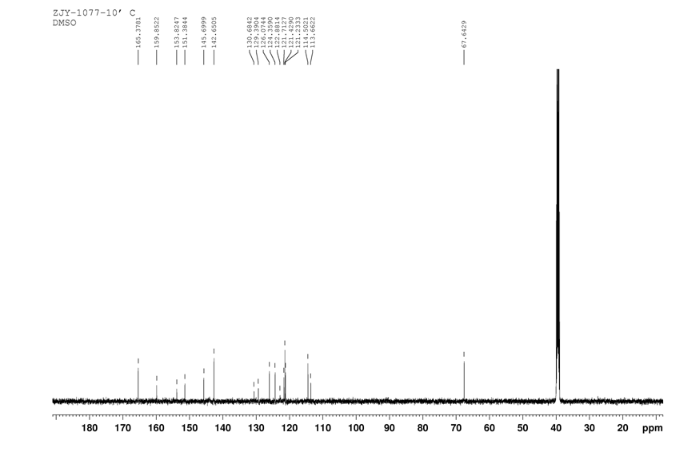
N4-(3-chloro-4-(thiazol-2-ylmethoxy)phenyl)quinazoline-4,6-diamine (11)
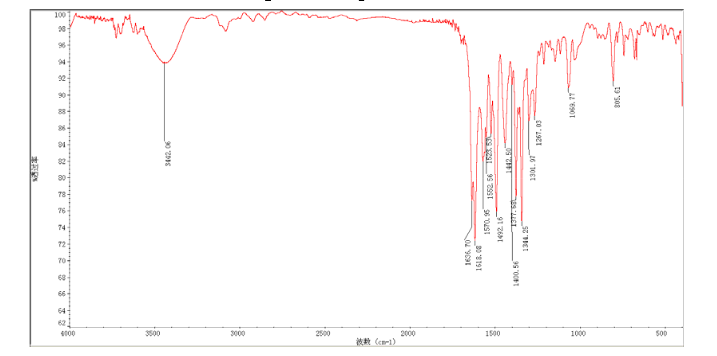
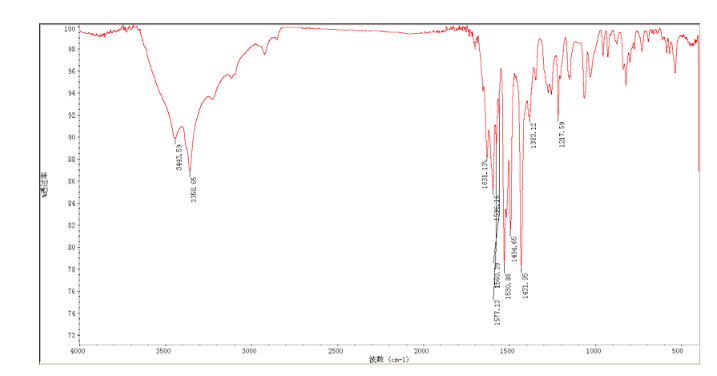
In a flask equipped with a reflux condenser, the compound 10 5.00 g (12.1 mmol), reduced zinc powder 3.2 g (48.5 mmo1, 4 eq), saturated ammonia chloride (3 mL) and methanol (60 mL) were mixed. The mixture was stirred at room temperature for 30 min. Then the zinc powder was filtered off, the filtrate was concentrated to obtain yellow solid 4.58 g, yield 98%. m.p. 197-198 °C (decompose); 1 H S4 NMR (DMSO-d6): δ 9.33(s, 1 H, exchangeable), 8.31 (s, 1 H), 8.05 (d, 1 H, J=2.6 Hz), 7.85 (d, 1 H, J=3.3 Hz), 7.79 (1 H, d, J=3.3 Hz), 7.73 (1 H, dd, J=2.5 Hz, J=9.0 Hz), 7.51 (1 H, d, J=8.9 Hz), 7.30 (1 H, d, J=2.4 Hz), 7.29 (1 H, d, J=4.7 Hz), 7.23 (1 H, dd, J=2.3 Hz, J=8.9 Hz), 5.57 (s, 2 H, exchangeable), 5.52 (s, 2 H); 13C NMR (DMSO-d6) δ: 165.9, 155.8, 149.7, 148.5, 147.3, 142.6, 142.5, 134.6, 128.7, 123.6, 123.2, 121.4, 121.3, 121.1, 116.5, 114. 7, 100.9, 67.8. IR (KBr) ν: 3443, 3358, 3211, 3100, 1631, 1596, 1577, 1560, 1530, 1494, 1431, 1383, 1217, 910 cm-1. Anal. calcd for C18H14N5OSCl: C 56.32, H 3.68, N 18.24, O 4.17; found C 56.34, H 3.70, N 18.22, O 4.14; MS (ESI) m/z: 382.66 (M-H).
Construction finally as per patent ……….US20050043334
Treatment of N4-[3-chloro-4-(thiazol-2-ylmethoxy)phenyl]quinazoline-4,6-diamine (11) with 1,1′-thiocarbonyldiimidazole , followed by condensation with 2(R)-amino-1-propanol in THF/CH2Cl2 affords thiourea derivative , which finally undergoes cyclization in the presence of TsCl and NaOH in THF/H2O to furnish varlitinib .
-
ASLAN Pharmaceuticals
-
Address: 10 Bukit Pasoh Rd, Singapore 089824Phone:+65 6222 4235


 carl fith
carl fith
Mr Carl Firth, CEO, Aslan Pharmaceuticals, Singapore (left) and Mr Dan Devine, CEO, Patrys, Australia (right)
///////ASLAN001, varlitinib, ASLAN Pharmaceuticals, Orphan Designation, ARRY-534, ARRY-334543 , PHASE 2, ORPHAN DRUG DESIGNATION, array
















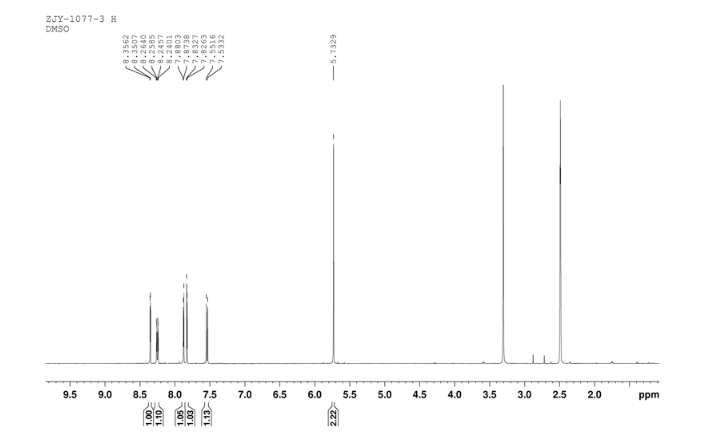
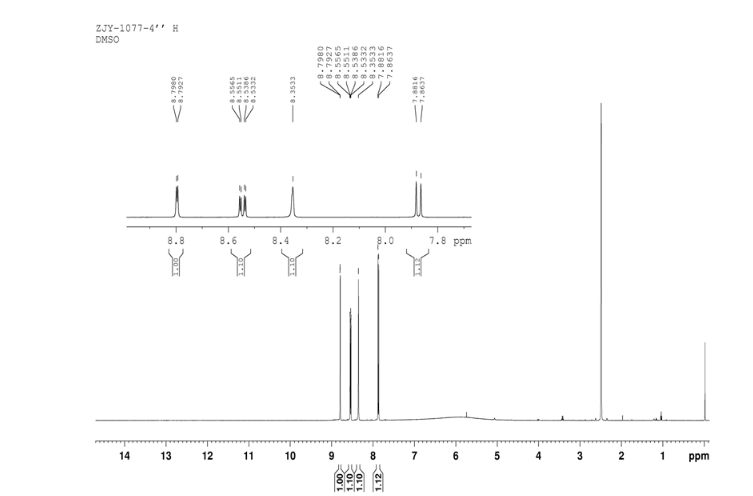 4 nmr dmsod6
4 nmr dmsod6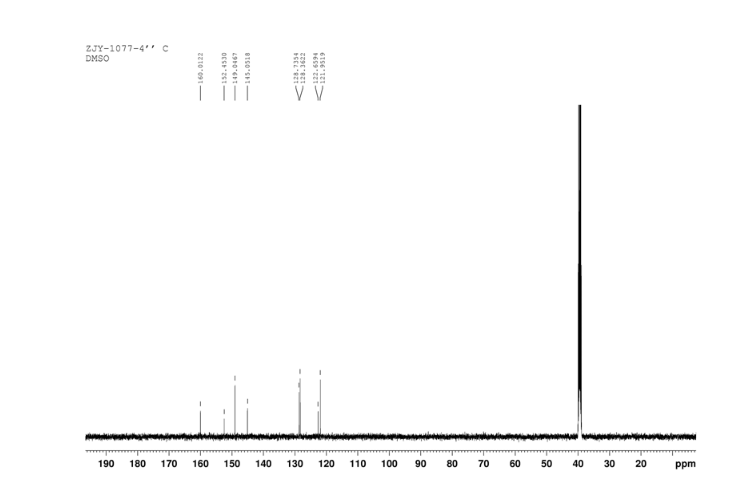
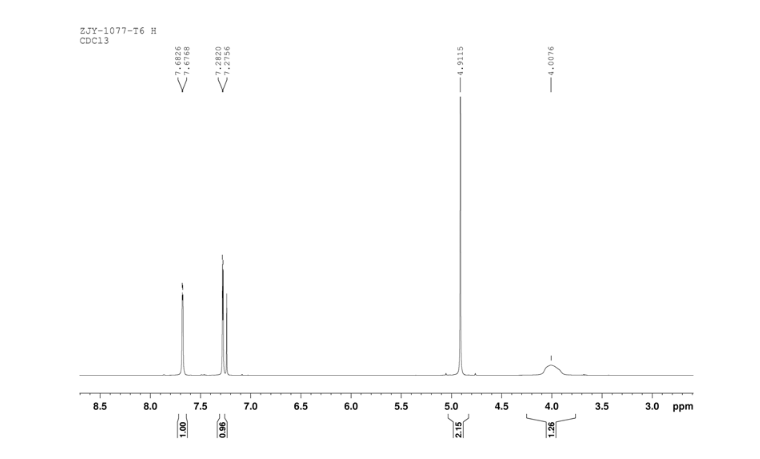 6 in dmsod6 1H NMR
6 in dmsod6 1H NMR