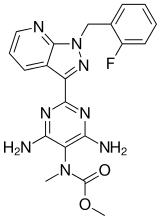
sumanirole
179386-43-7
179386-44-8 (maleate)

Sumanirole maleate, U-95666 (free base), U-95666E, PNU-95666E
| Process for synthesis of chiral 3-substituted tetrahydroquinoline derivatives | |
| Council Of Scientific & Industrial Research | |
| The present invention relates to novel and concise process for the construction of chiral 3-substituted tetrahydroquinoline derivatives based on proline catalyzed asymmetric α-functionalization of aldehyde, followed by in situ reductive cyclization of nitro group under catalytic hydrogenation condition with high optical purities. Further the invention relates to conversion of derived chiral 3-substituted tetrahydroquinoline derivatives into therapeutic agents namely (-)-sumanirole (96% ee) and 1-[(S)-3-(dimethylamino)-3,4-dihydro-6,7-dimethoxy-quinolin-1(2H)-yl]propanone[(S)-903] (92% ee). | |
| Process,sumanirole | |
| Indications | Restless legs syndrome; Parkinsons disease |
| Target-based Actions | Dopamine D2 receptor agonist |
| Other Actions | Anxiolytic; Antiparkinsonian |
 |
|
| Inventors | Boopathi, Senthil, Kumar; Arumugam, Sudalai; Rawat, Varun |
| IPC Codes | C07D 215/20; C07D 471/06; C07D 215/38 |
| DRUG | sumanirole |
| Publication Date | 26-Sep-2013 WO-2013140419-A1 |
Sumanirole (PNU-95,666) is a highly selective D2 receptor full agonist, the first of its kind to be discovered. It was developed for the treatment of Parkinson’s disease andrestless leg syndrome. While it has never been approved for medical use it is a highly valuable tool compound for basic research to identify neurobiological mechanisms that are based on a dopamine D2-linked (vs. D1, D3, D4, and D5-linked) mechanism of action
sumanirole
OTHER INFO

D-Phenylalanine (I) was protected as the methyl carbamate (II) by acylation with methyl chloroformate under Schotten-Baumann conditions. The N-methoxy amide (III) was then prepared by coupling of (II) with O-methyl hydroxylamine in the presence of EDC. Cyclization of (III) to the N-methoxy quinolinone (IV) was accomplished by treatment with bis(trifluoroacetoxy)iodobenzene in the presence of trifluoroacetic acid. Simultaneous reduction of the N-methoxy lactam and carbamate functions of (IV) by means of borane-methyl sulfide complex provided diamine (V). The aliphatic amino group of (V) was then selectively protected as the benzyl carbamate (VI) by using N-(benzyloxycarbonyloxy)succinimide at -40 C. Reaction of (VI) with phosgene, followed by treatment of the intermediate carbamoyl chloride with O-methyl hydroxylamine gave rise to the N-methoxy urea derivative (VII). This was cyclized with bis(trifluoroacetoxy)iodobenzene to the imidazoquinolinone (VIII). The N-methoxy and N-benzyloxycarbonyl groups of (VIII) were then removed by hydrogenolysis in the presence of Pearlman’s catalyst, and the title compound was finally converted to the corresponding maleate salt.
JOC 1997,62,(19):6582