
A group of American scientists developed microgels that release insulin in response to changing blood glucose levels
check these videos
…………
……….

FDA lifts clinical hold on Cangene’s hemophilia compound IB1001
WINNIPEG, July 29, 2013 /CNW/ – Cangene Corporation (Cangene) today announces that the U.S. Food and Drug Administration (FDA) has lifted the clinical hold previously placed on clinical trials evaluating the safety and efficacy of IB1001, a recombinant Factor IX (rFIX) product being developed for the treatment and prevention of bleeding episodes with hemophilia B.
http://www.pharmalive.com/fda-lifts-clinical-hold-on-cangenes-hemophilia-compoun
IB1001 is an intravenous recombinant Factor IX (rFIX) being developed for the treatment and prevention of bleeding episodes in people with hemophilia B. The IB1001 development program includes a comprehensive set of pharmacokinetic, safety, and efficacy data from a Phase 3 clinical trial in people affected by hemophilia B, including a surgery sub-study.
A biologics license application (BLA) was submitted to the U.S. Food and Drug Administration (FDA) in March 2012 and a Marketing Authorization Application (MAA) was submitted to the European Medicines Agency (EMA) in October 2011.
In July 2012, IB1001 was put on a clinical hold by the FDA due to a higher than expected rate of host cell antibody development in people treated with IB1001.
Cangene Corporation (TSX: CNJ) has completed its previously announced acquisition of the worldwide rights to investigational hemophilia compound IB1001 (recombinant FIX), as well as Inspiration’s rights to two product candidates in pre-clinical development: IB1007 (recombinant FVIIa) and IB1008 (recombinant FVIII) from Ipsen (Euronext: IPN; ADR: IPSEY) and Inspiration Biopharmaceuticals, Inc., in connection with Inspiration’s bankruptcy proceedings.
The transaction was slated to close on February 15, 2013.
Pursuant to the terms of the agreement, Cangene will pay approximately US $5.9 million upfront and up to $50 million in potential additional commercial milestones as well net sales payments equivalent to tiered double-digit percentage of IB1001 annual net sales.


U.S. drugmaker Perrigo agreed to buy Ireland’s Elan for $8.6 billion in a deal that should allow the rapidly growing company to reduce its tax bill and boost its royalty stream.

Perrigo Co. said it would pay Elan Corp.’s investors $6.25 per share in cash and $10.25 in Perrigo stock, an 11% premium over Elan’s closing price on July 26. Elan shares in Dublin surged 13% higher to 12.58 euros ($16.71), above Perrigo’s offer price, following news of the takeover.
http://www.dddmag.com/news/2013/07/perrigo-buying-elan-86b

Kiran Mazumdar-Shaw, chairman and managing director, Biocon.
Indian biopharma giant Biocon reported healthy growth of 22 percent for Q1 FY14 riding on the back of an increased geographical footprint in the emerging markets

Bangalore: Indian biopharma giant Biocon reported healthy growth of 22 percent for Q1 FY14. The firm clocked revenues worth $121 million (Rs723 crore), EBITDA of $29.50 million (Rs175 crore); and profit after tax (PAT) of $15.80 million (Rs94 crore).
Read more at: http://www.biospectrumasia.com/biospectrum/news/192549/how-biocon-clock-revenue-worth-usd121-mn#.UfdqX6I3CSo

Biocon’s India-focused branded formulations vertical as well as research services continue to grow at a steady pace


Health Canada has approved two GlaxoSmithKline (GSK) drugs, Tafinlar(TM) (dabrafenib mesilate) and Mekinist(TM) (trametinib), for patients with unresectable or metastatic melanoma. Dabrafenib mesylate, which is a BRAF-inhibitor, is indicated as a monotherapy oral treatment for unresectable melanoma or metastatic melanoma in adult …http://www.gsk.ca/english/html/media-centre/2013-07-24.html

Iron oxide nanoparticles precisely direct stem cells to damage tissues, thereby increasing their therapeutic potential
http://www.chemistryviews.org/details/news/5039301/Iron_Oxide_Nanoparticles_Show_the_Way.html
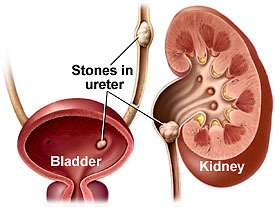
The discovery of a gene’s function in E. coli and other bacteria might lead to a probiotic to prevent calcium-oxalate urinary stones, the most common type of kidney stone. Human cells can’t metabolize oxalate and high concentrations can result in painful blockages of the urinary tract.