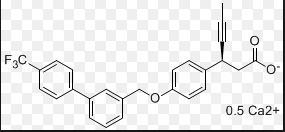
AMG 837
865231-46-5 (AMG-837 free acid); 865231-45-4 (AMG-837 sodium salt)
(S)-3-(4-((4′-(trifluoromethyl)-[1,1′-biphenyl]-3-yl)methoxy)phenyl)hex-4-ynoic acid
Description of AMG-837: AMG-837 is a potent, orally bioavailable GPR40 agonist. AMG 837 was a potent partial agonist in the calcium flux assay on the GPR40 receptor and potentiated glucose stimulated insulin secretion in vitro and in vivo. Acute administration of AMG 837 lowered glucose excursions and increased glucose stimulated insulin secretion during glucose tolerance tests in both normal and Zucker fatty rats. The improvement in glucose excursions persisted following daily dosing ofAMG 837 for 21-days in Zucker fatty rats. Preclinical studies demonstrated that AMG 837 was a potent GPR40 partial agonist which lowered post-prandial glucose levels. These studies support the potential utility of AMG 837 for the treatment of type 2 diabetes. (PLoS One. 2011;6(11):e27270).
Current developer: Amgen Inc

A new versatile method for the iridium-catalyzed asymmetric substitution of racemic allylic alcohols is exemplified by the depicted synthesis of AMG 837, a GPR40 receptor agonist that is of interest for the treatment of type 2 diabetes.
The allylic alkynylation (27 examples) typically provides excellent branched-to-linear regioselectivity (rr > 50:1) and high enantioselectivity (≥99%). The scope of the allylic alkynylation was explored using 12 allylic alcohols and 15 potassium alkynyltrifluoroborates.
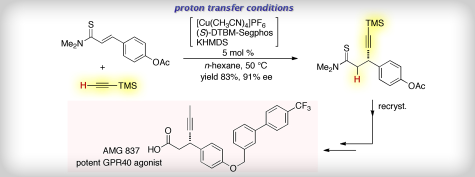
“Enantioselective Synthesis of a GPR40 Agonist AMG 837 via Catalytic Asymmetric Conjugate Addition of Terminal Alkyne to α,β-Unsaturated Thioamide”
Yazaki, R.; Kumagai, N.; Shibasaki, M.
Org. Lett. 2011, 13, 952. highlighted by Synfacts 2011, 6, 586.
PAPER
Scheme 18 Optimized preparation of biphenyl 54.
Scheme 17 Original Suzuki reaction employed for the synthesis of biphenyl 54.

http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532014001202186
/////////