PROCESS CHEMISTRY…DRUGS

Process chemistry is the arm of pharmaceutical chemistry concerned with the development and optimization of a synthetic scheme and pilot plant procedure to manufacture compounds for the drug development phase. Process chemistry is distinguished from medicinal chemistry, which is the arm of pharmaceutical chemistry tasked with designing and synthesizing molecules on small scale in the early drug discovery phase.

Medicinal chemists are largely concerned with synthesizing a large number of compounds as quickly as possible from easily tunable chemical building blocks (usually for SAR studies). In general, the repertoire of reactions utilized in discovery chemistry is somewhat narrow (for example, the Buchwald-Hartwig amination, Suzuki coupling and reductive amination are commonplace reactions).[1] In contrast, process chemists are tasked with identifying a chemical process that is safe, cost and labor efficient, “green,” and reproducible, among other considerations.
Oftentimes, in searching for the shortest, most efficient synthetic route, process chemists must devise creative synthetic solutions that eliminate costly functional group manipulations and oxidation/reduction steps.
This article will focus exclusively on the chemical and manufacturing processes associated with the production of small molecule drugs. Biological medical products (more commonly called “biologics”) represent a growing proportion of approved therapies, but the manufacturing processes of these products are beyond the scope of this article.
Additionally, the many complex factors associated with chemical plant engineering (for example, heat transfer and reactor design) and drug formulation will be treated cursorily.

Process Chemistry Considerations
Cost efficiency is of paramount importance in process chemistry and, consequently, is a focus in the consideration of pilot plant synthetic routes. The drug substance that is manufactured, prior to formulation, is commonly referred to as the active pharmaceutical ingradient (API) and will be referred to as such herein.
API production cost can be broken into two components: the “material cost” and the “conversion cost.”[2] The ecological and environmental impact of a synthetic process should also be evaluated by an appropriate metric (e.g. the EcoScale).
An ideal process chemical route will score well in each of these metrics, but inevitably tradeoffs are to be expected. Most large pharmaceutical process chemistry and manufacturing divisions have devised weighted quantitative schemes to measure the overall attractiveness of a given synthetic route over another. As cost is a major driver, material cost and volume-time output are typically weighted heavily.
The chemical and processing industries (CPI) provide the building blocks for many products. By using large amounts of heat and energy to physically or chemically transform materials, these industries help meet the world’s most fundamental needs for food, shelter and health, as well as products that are vital to such advanced technologies as computing, telecommunications and biotechnology.
These industries face major challenges to meet the needs of the present without compromising the needs of the future generations in the face of increasing industrial competitiveness. This translates into the need to make processes much more energy efficient, safer and more flexible, and to reduce emissions to meet the many competitive challenges within a global economy.
The chemical and processing industries refer to processes where materials undergo chemical conversion during their production into finished products, as well as – or instead of – the physical conversions common to industry in general.
In the chemical process industry the products differ chemically from the raw materials as a result of undergoing one or more chemical reactions during the manufacturing process.
The chemical process industries broadly include the traditional chemical industries, both organic and inorganic; the petroleum industry; the petrochemical industry, which produces the majority of plastics, synthetic fibers, and synthetic rubber from petroleum and natural-gas raw materials; and a series of allied industries in which chemical processing plays a substantial part.
While the chemical process industries are primarily the realm of the chemical engineer and the chemist, they also involve a wide range of other scientific, engineering, and economic specialists.

Material Cost
The material cost of a chemical process is the sum of the costs of all raw materials, intermediates, reagents, solvents and catalysts procured from external vendors. Material costs may influence the selection of one synthetic route over another or the decision to outsource production of an intermediate.
Conversion Cost
The conversion cost of a chemical process is a factor of that procedure’s overall efficiency, both in materials and time, and its reproducibility. The efficiency of a chemical process can be quantified by its atom economy, yield, volume-time output, and environmental factor (E-factor), and its reproducibility can be evaluated by the Quality Service Level (QSL) and Process Excellence Index (PEI) metrics.
Atom Economy
The atom economy of a reaction is defined as the number of atoms from the starting materials that are incorporated into the final product. Atom economy can be viewed as an indicator of the “efficiency” of a given synthetic route.[3]

For example, the Claisen rearrangement and the Diels-Alder cycloaddition are examples of reaction that are 100 percent atom economical. On the other hand, a prototypical Wittig reaction has especially poor atom economy (merely 20 percent in the example shown).
Process synthetic routes should be designed such that atom economy is maximized for the entire synthetic scheme. Consequently, “costly” reagents such as protecting groups and high molecular weight leaving groups should be avoided where possible. An atom economy value in the range of 70 to 90 percent for an API synthesis is ideal, but it may be impractical or impossible to access certain complex targets within this range. Nevertheless, atom economy is a good metric to compare two routes to the same molecule.
Yield
Yield is defined as the amount of product obtained in a chemical reaction. According to Vogel’s Textbook of Practical Organic Chemistry, yields around 100% are called quantitative, yields above 90% are excellent, yields above 80% are very good, yields above 70% are good, yields above 50% are fair, and yields below 40% are poor. The yield that has practical significance in a process chemistry setting is the isolated yield, referring to the yield of the isolated product after all extraction and purification steps. In a final API synthesis, isolated yields of 80 percent or above for each synthetic step are expected.
There are several strategies that are employed in the design of a process route to ensure adequate overall yield of the pharmaceutical product. The first is the concept of convergent synthesis. Assuming a very good to excellent yield in each synthetic step, the overall yield of a multistep reaction can be maximized by combining several key intermediates at a late stage that are prepared independently from each other.
Another strategy to maximize isolated yield (as well as time efficiency) is the concept of telescoping synthesis (also called one-pot synthesis). This approach describes the process of eliminating workup and purification steps from a reaction sequence, typically by simply adding reagents sequentially to a reactor. In this way, unnecessary losses from these steps can be avoided.
Finally, to minimize overall cost, synthetic steps involving expensive reagents, solvents or catalysts should be designed into the process route as late stage as possible, to minimize the amount of reagent used.
In a pilot plant or manufacturing plant setting, yield can have a profound effect on the material cost of an API synthesis, so the careful planning of a robust route and the fine-tuning of reaction conditions are crucially important. After a synthetic route has been selected, process chemists will subject each step to exhaustive optimization in order to maximize overall yield. Low yields are typically indicative of unwanted side product formation, which can raise red flags in the regulatory process as well as pose challenges for reactor cleaning operations.
Volume-Time Output
The volume-time output (VTO) of a chemical process represents the cost of occupancy of a chemical reactor for a particular process or API synthesis. For example, a high VTO indicates that a particular synthetic step is costly in terms of “reactor hours” used for a given output. Mathematically, the VTO for a particular process is calculated by the total volume of all reactors (m3) that are occupied times the hours per batch divided by the output for that batch of API or intermediate (measured in kg).
![VTO=\frac{\text{nominal volume of all reactors} [m^3]*\text{time per batch} [h]}{\text{output per step} [kg]}](http://upload.wikimedia.org/math/7/9/a/79aca4fd95405c01c04df9181fb3ee63.png)
The process chemistry group at Boehringer-Ingelheim, for example, targets a VTO of less than 1 for any given synthetic step or chemical process.
Additionally, the raw conversion cost of an API synthesis (in dollars per batch) can be calculated from the VTO, given the operating cost and usable capacity of a particular reactor. Oftentimes, for large-volume APIs, it is economical to build a dedicated production plant rather than to use space in general pilot plants or manufacturing plants.
Environmental Factor (E-factor) and Process Mass Intensity (PMI)
Both of these measures, which capture the environmental impact of a synthetic reaction, intend to capture the significant and rising cost of waste disposal in the manufacturing process. The E-factor for an entire API process is computed by the ratio of the total mass of waste generated in the synthetic scheme to the mass of product isolated.

A similar measure, the process mass intensity (PMI) calculates the ratio of the total mass of materials to the mass of the isolated product.

For both metrics, all materials used in all synthetic steps, including reaction and workup solvents, reagents and catalysts, are counted, even if solvents or catalysts are recycled in practice. Inconsistencies in E-factor or PMI computations may arise when choosing to consider the waste associated with the synthesis of outsourced intermediates or common reagents. Additionally, the environmental impact of the generated waste is ignored in this calculation; therefore, the environmental quotient (EQ) metric was devised, which multiplies the E-factor by an “unfriendliness quotient” associated with various waste streams. A reasonable target for the E-factor or PMI of a single synthetic step is any value between 10 and 40.
Quality Service Level (QSL)
The final two “conversion cost” considerations involve the reproducibility of a given reaction or API synthesis route. The quality service level (QSL) is a measure of the reproducibility of the quality of the isolated intermediate or final API. While the details of computing this value are slightly nuanced and unimportant for the purposes of this article, in essence, the calculation involves the ratio of satisfactory quality batches to the total number of batches. A reasonable QSL target is 98 to 100 percent.
Process Excellence Index (PEI)
Like the QSL, the process excellence index (PEI) is a measure of process reproducibility. Here, however, the robustness of the procedure is evaluated in terms of yield and cycle time of various operations. The PEI yield is defined as follows:
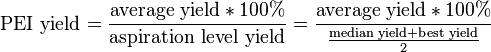
In practice, if a process is high-yielding and has a narrow distribution of yield outcomes, then the PEI should be very high. Processes that are not easily reproducible may have a higher aspiration level yield and a lower average yield, lowering the PEI yield.
Similarly, a PEI cycle time may be defined as follows:

For this expression, the terms are inverted to reflect the desirability of shorter cycle times (as opposed to higher yields). The reproducibility of cycle times for critical processes such as reaction, centrifugation or drying may be critical if these operations are rate-limiting in the manufacturing plant setting. For example, if an isolation step is particularly difficult or slow, it could become the bottleneck for an API synthesis, in which case the reproducibility and optimization of that operation become critical.
For an API manufacturing process, all PEI metrics (yield and cycle times) should be targeted at 98 to 100 percent.
EcoScale
In 2006, Van Aken, et al.[4] developed a quantitative framework to evaluate the safety and ecological impact of a chemical process, as well as minor weighting of practical and economical considerations. Others have modified this EcoScale by adding, subtracting and adjusting the weighting of various metrics. Among other factors, the EcoScale takes into account the toxicity, flammability and explosive stability of reagents used, any nonstandard or potentially hazardous reaction conditions (for example, elevated pressure or inert atmosphere), and reaction temperature. Some EcoScale criteria are redundant with previously considered criteria (e.g. E-factor).
Synthetic Case Studies
Boehringer Ingelheim HCV Protease Inhibitor (BI 201302)
Macrocyclization is a recurrent challenge for process chemists, and large pharmaceutical companies have necessarily developed creative strategies to overcome these inherent limitations. An interesting case study in this area involves the development of novel NS3 protease inhibitors to treat Hepatitis C patients by scientists at Boehringer-Ingelheim.[5] The process chemistry team at BI was tasked with developing a cheaper and more efficient route to the active NS3 inhibitor BI 201302, a close analog of BILN 2061. Two significant shortcomings were immediately identified with the initial scale-up route to BILN 2061, depicted in the scheme below.[6] The macrocyclization step posed four challenges inherent to the cross-metathesis reaction.
- High dilution is typically necessary to prevent unwanted dimerization and oligomerization of the diene starting material. In a pilot plant setting, however, a high dilution factor translates into lower throughput, higher solvent costs and higher waste costs.
- High catalyst loading was found to be necessary to drive the RCM reaction to completion. Because of high licencing costs of the ruthenium catalyst that was used (1st generation Hoveyda catalyst), a high catalyst loading was financially prohibitive. Recycling of the catalyst was explored, but proved impractical.
- Long reaction times were necessary for reaction completion, due to slow kinetics of the reaction using the selected catalyst. It was hypothesized that this limitation could be overcome using a more active catalyst. However, while the second-generation Hoveyda and Grubbs catalysts were kinetically more active than the first-generation catalyst, reactions using these catalysts formed large amounts of dimeric and oligomeric products.
- An epimerization risk under the cross-methathesis reaction conditions. The process chemistry group at Boehringer-Ingelheim performed extensive mecahnistic studies showing that epimerization most likely occurs through a ruthenacyclopentene intermediate.[7] Furthermore, the Hoveyda catalyst employed in this scheme minimizes epimerization risk compared with the alalogous Grubbs catalyst.
Additionally, the final double SN2 sequence to install the quinoline heterocycle was identified as a secondary inefficiency in the synthetic route.
Analysis of the cross-methathesis reaction revealed that the conformation of the acyclic precursor had a profound impact on the formation of dimers and oligomers in the reaction mixture. By installing a Boc protecting group at the C-4 amide nitrogen, the Boehringer-Ingelheim chemists were able to shift the site of initiation from the vinylcyclopropane moiety to the nonenoic acid moiety, improving the rate of the intramolecular reaction and decreasing the risk of epimerization. Additionally, the catalyst employed was switched from the expensive 1st generation Hoveyda catalyst to the more reactiveless expensive Grela catalyst.[8] These modifications allowed the process chemists to run the reaction at a standard reaction dilution of 0.1-0.2 M, given that the rates of competing dimerization and oligomerization reactions was so dramatically reduced.
Additionally, the process chemistry team envisioned a SNAr strategy to install the quinoline heterocycle, instead of the SN2 strategy that they had employed for the synthesis of BILN 2061. This modification prevented the need for inefficient double inversion by proceeding through retention of stereochemistry at the C-4 position of the hydroxyproline moiety.[9]
It is interesting to examine this case study from a VTO perspective. For the unoptimized cross-metathesis reaction using the Grela catalyst at 0.01 M diene, the reaction yield was determined to be 82 percent after a reaction and workup time of 48 hours. A 6-cubic meter reactor filled to 80% capacity afforded 35 kg of desired product. For the unoptimized reaction:
This VTO value was considered prohibitively high and a steep investment in a dedicated plant would have been necessary even before launching Phase III trials with this API, given its large projected annual demand. But after reaction development and optimization, the process team was able to improve the reaction yield to 93 percent after just 1 hour (plus 12 hours for workup and reactor cleaning time) at a diene concentration of 0.2 M. With these modifications, a 6-cubic meter reactor filled to 80% capacity afforded 799 kg of desired product. For this optimized reaction:
Thus, after optimization, this synthetic step became less costly in terms of equipment and time and more practical to perform in a standard manufacturing facility, eliminating the need for a costly investment in a new dedicated plant.
Simvastatin, originally developed by Merck, is the most frequently prescribed statin today, with more nearly 100 million prescriptions filled in 2010, according to IMS Health. The traditional synthesis of the drug entailed a multi-step chemical process starting from Lovastatin. The chemical process was using large amounts of hazardous reagents as well as large quantities of solvents.
Professor Yi Tang, at UCLA conceived an initial synthesis that used an engineered enzyme. Codexis Inc. licensed the intellectual property from UCLA, optimized the initial enzyme and developed the new process for commercial use as shown in Figure 1. Following the quantitative hydrolysis of lovastatin to monacolin J acid, Codexis developed a novel, non-natural acyl donor enzyme to regioselectively acylate the C8 position and effect cyclization to simvastatin. This mild bioenzymatic process reduces the 4 steps chemical synthesis to only two steps. The Codexis process is significantly more efficient, cost effective and environmentally friendly.

This is the reaction scheme for producing the drug Simvastatin. The process was an award-winner at last month’s Green Chemistry Challenge Awards held by the Environmental Protection Agency
“We started working on Simvastatin in 2008 and completed the planning process in 2010,” Huisman said. “Then, we started the commercialization process, which takes time because you need regulatory approval of the new process we were working on. We licensed some technology from Yi Tang and UCLA and were then able to continue.”
Codexis took the three-step process used to make Simvastatin and cut out two of the steps, Huisman said.
“From the starting material, it (Simvastatin) has three reactive groups, or hydroxy groups, and what we need to do is convert two of the three groups,” Huisman explained. “We took out a protective step and a de-protective step. We took out two of the steps, and it was intense chemical processing. We then were able to accomplish everything in one step. We also circumvented the use of several nasty chemicals, as well.”
By cutting out two steps, “the overall yield goes up tremendously, about 35 percent,” Huisman added. “And we’re generating 25 times less waste than we did in the old process.”
Huisman said the new process doesn’t change the drug’s effects at all, and that scientists have been trying to do this type of work on commercial drugs for decades.
“In order for this to be a commercial process, the enzyme needs to be improved,” he said. “We needed to speed up the enzyme 1,000-fold to make this process workable; it took a team of scientists about nine months to optimize the enzymes and speed it up.”

Codexis 2 step enzymatic process versus the 4 step chemical synthesis
AZIDES
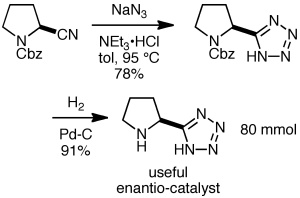
A popular procedure for making 5-substituted tetrazoles is the reaction of sodium azide with a nitrile, often in the presence of an ammonium salt. The example shown below is from Organic Syntheses (Novartis Process R&D and Ley’s group at Cambridge), providing the useful enantiocatalyst shown on an 80 mmol scale. The excess sodium azide was destroyed with sodium nitrite and sulfuric acid, which converts hydrazoic acid into nitrogen and nitrous oxide gases.
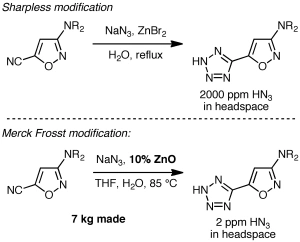
While the above procedure may be popular, any time you use sodium azide you should be thinking, “hydrazoic acid can be generated, it’s explosive and toxic, and I need to take the appropriate safety precautions.” That’s precisely what happened during some recent process R&D work at Merck Frosst on the steroyl-CoA desaturase inhibitor MK-8245. The discovery chemistry route used NaN3/pyridinium chloride as shown below, but the process group felt that the potential for significant amounts of hydrazoic acid generation was too high.
Armed with the ability to detect hydrazoic acid in the headspace above the reaction mixture using online IR, the Merck Frosst researchers surveyed alternatives. Sharpless’s zinc bromide procedure, proposed to minimize hydrazoic acid formation by control of the pH, led to a reading of 2000 ppm of HN3 in the headspace, which is below the detonation threshold of 15,000 ppm but was still felt to be undesirable. In their own survey of conditions, the Merck Frosst scientists found something quite new and significant: Reaction with sodium azide in the presence of a catalytic amount of zinc oxide in aqueous THF (pH 8) proceeded efficiently, and most notably, with only 2 ppm of HN3 in the headspace! They were able to make 7 kg of the tetrazole in one run in nearly quantitative yield. Nice!
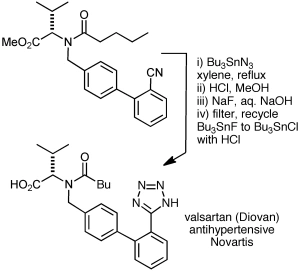
I’d be remiss if I didn’t mention Bu3SnN3 and Me3SiN3/Cu(I) as sodium azide surrogates, sometimes used on large scale. Shown below is an application to valsartan (see here and here) with recycling of the tin by-products. The intermediate stannyl tetrazole and leftover Bu3SnN3 were converted with HCl to Bu3SnCl, which was then converted to the fluoride, which was removed by filtration and recycled to Bu3SnCl.
Additional Topics
Transition-Metal Catalysis and Organocatalysis
Biocatalysis and Enzymatic Engineering
Recently, large pharmaceutical process chemists have relied heavily on the development of enzymatic reactions to produce important chiral building blocks for API synthesis. Many varied classes of naturally occurring enzymes have been co-opted and engineered for process pharmaceutical chemistry applications. The widest range of applications come from ketoreductases and transaminases, but there are isolated examples from hydrolases, aldolases, oxidative enzymes, esterases and dehalogenases, among others.[10]
One of the most prominent uses of biocatalysis in process chemistry today is in the synthesis of Januvia®, a DPP-4 inhibitor developed by Merck for the management of type II diabetes. The traditional process synthetic route involved a late-stage enamine formation followed by rhodium-catalyzed asymmetric hydrogenation to afford the API sitagliptin. This process suffered from a number of limitations, including the need to run the reaction under a high-pressure hydrogen environment, the high cost of a transition-metal catalyst, the difficult process of carbon treatment to remove trace amounts of catalyst and insufficient stereoselectivity, requiring a subsequent recrystallization step before final salt formation.[11][12]
Merck’s process chemistry department contracted Codexis, a medium-sized biocatalysis firm, to develop a large-scale biocatalytic reductive amination for the final step of its sitagliptin synthesis. Codexis engineered a transaminase enzyme from the bacteria Arthrobacter through 11 rounds of directed evolution. The engineered transaminase contained 27 individual point mutations and displayed activity four orders of magnitude greater than the parent enzyme. Additionally, the enzyme was engineered to handle high substrate concentrations (100 g/L) and to tolerate the organic solvents, reagents and byproducts of the transamination reaction. This biocatalytic route successfully avoided the limitations of the chemocatalyzed hydrogenation route: the requirements to run the reaction under high pressure, to remove excess catalyst by carbon treatment and to recrystallize the product due to insufficient enantioselectivity were obviated by the use of a biocatalyst. Merck and Codexis were awarded the Presidential Green Chemistry Challenge Award in 2010 for the development of this biocatalytic route toward Januvia®.[13]

ATORVASTATIN
Biocatalytic process development firm Codexis was recognized with the award in the greener reaction conditions category for developing a “green-by-design” enzymatic process to replace a chemical process for making ethyl (R)-4-cyano-3-hydroxybutyrate. This chemical, also known as hydroxynitrile, is the key chiral building block used to make atorvastatin, the active ingredient in Pfizer‘s cholesterol-lowering drug Lipitor.
The new process is helping to lower atorvastatin’s long-term production costs, according to John H. Grate, senior vice president of R&D and chief technology officer at Codexis. The savings could be financially significant for Pfizer and future generics manufacturers given that Lipitor is the world’s top pharmaceutical, with annual sales of about $13 billion.

Hydroxynitrile is used in the early stages of atorvastatin synthesis to build the chiral dihydroxy acid side chain that’s essential to the drug’s activity, Grate told C&EN. Demand for the intermediate is about 200 metric tons per year, and it’s currently being made by several fine chemicals producers. The competition to supply the intermediate to Pfizer has spurred several firms to chase after a better way to prepare hydroxynitrile (Angew. Chem. Int. Ed. 2005, 44, 362).

Chemical engineering professor Galen J. Suppes of the University of Missouri, Columbia, was honored with the academic award for his group’s work to create a low-cost catalytic process to convert the glycerol by-product from biodiesel production into propylene glycol–turning 1,2,3-propanetriol into 1,2-propanediol. At first glance, this achievement may not sound that exciting. But the repercussions of Suppes’s accomplishment are expected to have a major impact on the future use of biodiesel fuel, the world glycerol market, and the environmental health and safety of antifreeze and deicing chemicals.
 Photo by Rob Hill/MU Publications Photo by Rob Hill/MU Publications |
| GREEN SOLUTION Suppes and his group uncovered ideal reaction conditions for the catalytic conversion of by-product glycerol to useful propylene glycol. |
Biodiesel is a mixture of fatty acid methyl esters made by esterifying soybean oil or other vegetable oil or animal fat. The triglycerides in the oil consist of three long fatty acid chains connected to a propyl headgroup. Sodium hydroxide is used to cleave the chains, which in turn are reacted with methanol to form methyl esters, leaving the residual glycerol headgroup as a by-product. About 1 kg of crude glycerol is formed for every 9 kg of biodiesel produced.
Millions of gallons of glycerol are flooding the world market as biodiesel production is ramping up in the U.S. and Europe, Suppes explained. The fallout from this glycerol glut is that chemical companies have shuttered some glycerol production plants and are considering glycerol as a starting material to make a host of feedstock chemicals (C&EN, Feb. 6, page 7).
Suppes entered the picture about four years ago when he realized that an inexpensive method to convert glycerol to propylene glycol could be valuable, he said. Utilizing the glycerol not only would help offset the cost of biodiesel production, but the inexpensive propylene glycol could be used as a low-toxicity replacement for ethylene glycol in automotive antifreeze.
Suppes’s system involves low-pressure hydrogenolysis of glycerol using a copper chromite catalyst, CuO•Cr2O3 (Appl. Catal. A 2005, 281, 225). In the two-step process, glycerol is first dehydrated to form acetol (1-hydroxy-2-propanone), which is then hydrogenated to form propylene glycol.
 |
| GREEN LEFTOVERS Glycerol by-product from biodiesel production can be used as a feedstock in Suppes’ process to produce acetol or propylene glycol from renewable resources. |
Copper chromite hydrogenolysis catalysts aren’t new, but the success of the Missouri process is in achieving high selectivity for propylene glycol by controlling the temperature and hydrogen pressure of the reaction, Suppes noted. In the past, researchers tended to use reaction temperatures that were too high, leading to a higher percentage of by-products. Thus, they “missed the window of opportunity to achieve high selectivity,” Suppes said. Tinkering with temperature, pressure, and several different catalysts, Suppes and his colleagues optimized the system to operate at about 220 °C and less than 10 bar versus about 260 °C and more than 150 bar for other systems.
Another key part of the synthesis is the ability to isolate the acetol intermediate, Suppes added. Acetol is a synthetic starting material used to make polyols. But when made from petroleum, it costs about $5.00 per lb, discouraging its widespread use. Suppes envisions that producing acetol from biomass-based glycerol using his process could lower the cost to 50 cents per lb, “opening up even more potential applications and markets for products made from glycerol.”
Suppes’s propylene glycol process has been patented and is being licensed through the Missouri Soybean Merchandising Council, which provided partial funding for the research. The first commercial facility, with an annual capacity of 11.5 million gal, is being built in an undisclosed location in the U.S. by Senergy Chemical. It’s expected to be in operation by the end of this year.

E7398, INN eribulin mesylate
The most awe-inspiring example of a positive tangible outcome from the combination of basic research into the synthesis of a system, and a correctly weighted assessment of ‘scalability’, is Halaven® (2, E7398, INN eribulin mesylate). Most chemists in industry and academia alike would have considered using total synthesis to support clinical development and commercialization of this compound a ‘fool’s errand,’ but the Kishi group and Eisai Inc. did not. The fact is that this compound solves a major clinical problem, so taking on the issues (length of synthesis, stability limitations, stereochemical problems, etc.) had a big payoff (reducing the relative weighting or importance of these factors in assessing the viability of a commercial chemical synthesis). As depicted below, a highly convergent approach, combined with powerful methodology for stitching together key fragments 5 and 6 (Nozaki–Hiyama–Kishi (NHK) coupling) and a strategy of targeting crystalline intermediates were all key elements that culminated in this landmark accomplishment
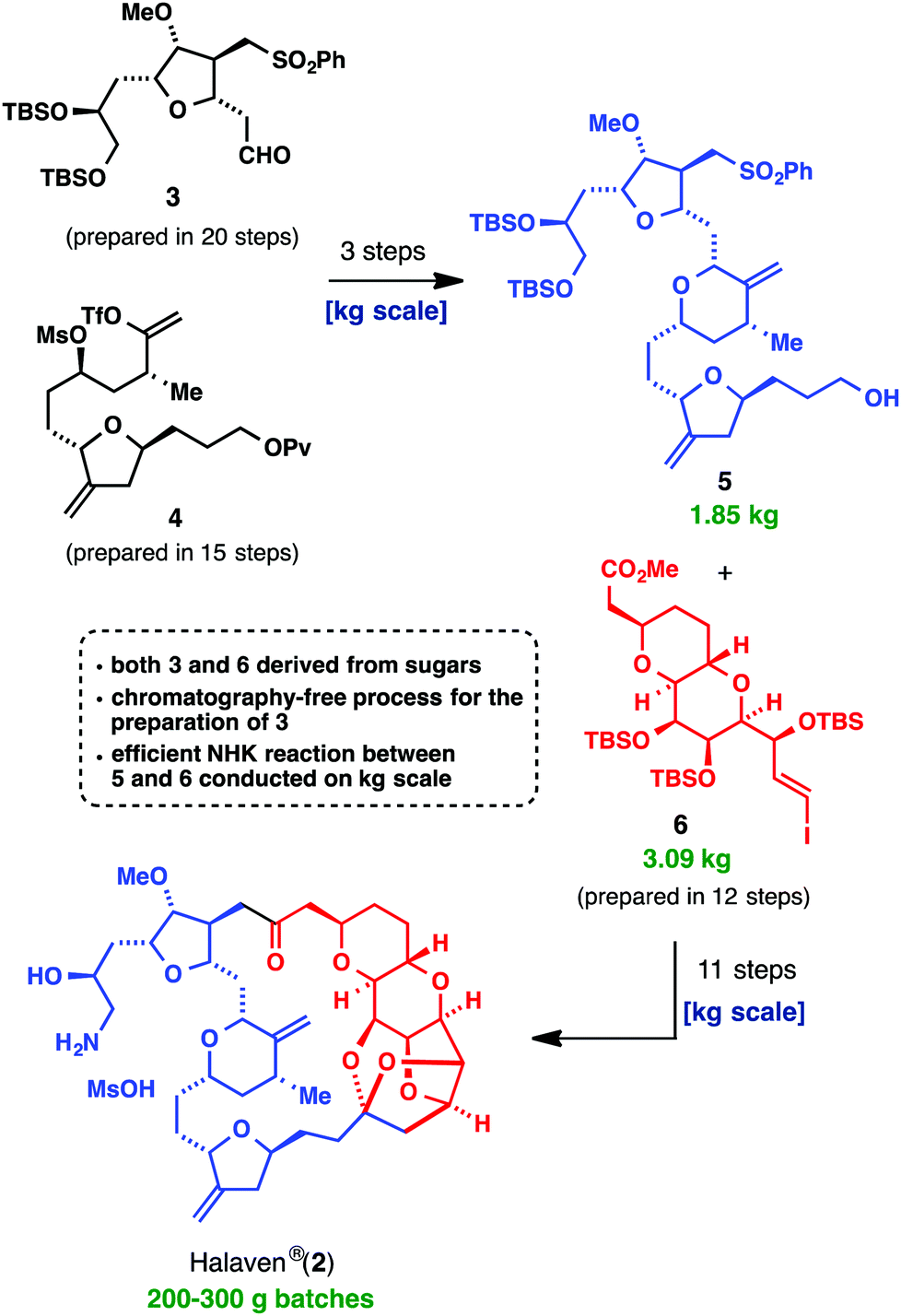
The commercial synthesis of Halaven® (2), a landmark achievement in process chemistry
Artemisinin (Cook, 2012).
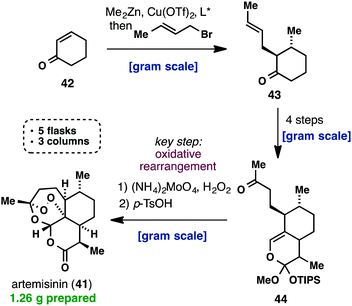
(+)-Artemisinin (41) is currently the most effective drug against Plasmodium falciparum malaria as part of an artemisinin-based combination therapy (ACT). Although it can be isolated on an industrial scale from Artemisia annua, the market price of artemisinin (41) has fluctuated widely and traditional extraction does not provide enough material to meet the worldwide demand. Interestingly, recent efforts towards a cheaper and more efficient production of artemisinin (41) have mainly taken place in the areas of synthetic biology, semisynthesis and plant engineering, while there has been a lack of practical approaches using a straightforward total synthesis. Despite the fact that all the total syntheses of artemisinin, until 2010, were impressive from a feasibility point of view, none of them provided a solution for the low-cost synthesis of 41. This changed when Cook’s group recently published a scalable synthesis of artemisinin (41), which provides a blueprint for the cost-effective production of 41 and its derivatives below Key to their successful strategy was the use of reaction cascades that rapidly built complexity, starting from the cheap feedstock chemical, cyclohexenone (42). The latter was first subjected to a one-pot conjugate addition/alkylation sequence, to give ketone 43. A three-step sequence consisting of formylation, cycloaddition and a Wacker-type oxidation, yielded 9.4 g of methyl ketone 44. The challenging formation of the unusual peroxide bridge was initially met with failure, but was eventually realized by a reaction with singlet oxygen to give 41 amongst other oxidized intermediates. The entire synthetic sequence was conducted on a gram scale, required only three chromatographic purifications and was carried out in only five flasks. Considering the low cost of the commodity chemicals used and the conciseness of Cook’s synthesis, it is certainly worth being further investigated.
Continuous/Flow Manufacturing
In recent years, much progress has been made in the development and optimization of flow reactors for small-scale chemical synthesis (the Jamison Group at MIT and Ley Group at Cambridge University, among others, have pioneered efforts in this field). The pharmaceutical industry, however, has been slow to adopt this technology for large-scale synthetic operations. For certain reactions, however, continuous processing may possess distinct advantages over batch processing in terms of safety, quality and throughput.
A case study of particular interest involves the development of a fully continuous process by the process chemistry group at Eli Lilly and Company for an asymmetric hydrogenation to access a key intermediate in the synthesis of LY500307,[14] a potent ERβ agonist that is entering clinical trials for the treatment of patients with schizophrenia, in addition to a regimen of standard antipsychotic medications. In this key synthetic step, a chiral rhodium-catalyst is used for the enantioselective reduction of a tetrasubstituted olefin. After extensive optimization, it was found that in order to reduce the catalyst loading to a commercially practical level, the reaction required hydrogen pressure up to 70 atm. The pressure limit of a standard chemical reactor is about 10 atm, although high-pressure batch reactors may be acquired at significant capital cost for reactions up to 100 atm. Especially for an API in the early stages of chemical development, such an investment clearly bears a large risk.
An additional concern was that the hydrogenation product has an unfavorable eutectic point, so it was impossible to isolate the crude intermediate in more than 94 percent ee by batch process. Because of this limitation, the process chemistry route toward LY500307 necessarily involved a kinetically controlled crystallization step after the hydrogenation to upgrade the enantiopurity of this penultimate intermediate to >99 percent ee.
The process chemistry team at Eli Lilly successfully developed a fully continuous process to this penultimate intermediate, including reaction, workup and kinetically controlled crystallization modules (the engineering considerations implicit in these efforts are beyond the scope of this article). An advantage of flow reactors is that high-pressure tubing can be utilized for hydrogenation and other hyperbaric reactions. Because the head space of a batch reactor is eliminated, however, many of the safety concerns associated with running high-pressure reactions are obviated by the use of a continuous process reactor. Additionally, a two-stage mixed suspension-mixed product removal (MSMPR) module was designed for the scalable, continuous, kinetically controlled crystallization of the product, so it was possible to isolate in >99 percent ee, eliminating the need for an additional batch crystallization step.
This continuous process afforded 144 kg of the key intermediate in 86 percent yield, comparable with a 90 percent isolated yield using the batch process. This 73-liter pilot-scale flow reactor (occupying less than 0.5 m3 space) achieved the same weekly throughput as theoretical batch processing in a 400-liter reactor. Therefore, the continuous flow process demonstrates advantages in safety, efficiency (eliminates the need for batch crystallization) and throughput, compared with a theoretical batch process.
US scientists have found a way to stop solid byproducts clogging channels in continuous flow reactors, a problem that has hampered their progress for use in manufacturing pharmaceuticals.
Klavs Jensen, Stephen Buchwald and their team at the Massachusetts Institute of Technology believe that flow methods will become increasingly important in the future of pharmaceuticals and chemical manufacturing. ‘One of the biggest hurdles is handling solids,’ says group member Timothy Noël. ‘Precipitates can form during the reactions, which usually lead to irreversible clogging of microchannels in the reactors.’ Previous methods suggested to overcome this problem include introducing another solvent to dissolve the solids, but this can reduce the overall efficiency of the reactions. Now, the team have used an ultrasound bath to break up the byproducts to prevent clogging.
Traditionally, pharmaceutical manufacture is done in a batch-based system, but the process suffers from interruptions and the need to transport material between batch reactors. Performing these reactions in a continuous flow system would speed up the process and reduce chemical waste.
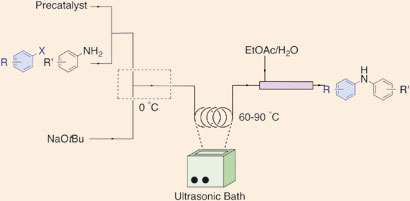
|
Reagents were introduced into a tube, which was then placed in an ultrasonic bath heated to 60 degrees Celsius. When the reagents exited the reactor, the reaction was mixed with a quench of water and ethyl acetate in a larger tube, allowing plenty of time for salt byproducts to dissolve
|
The team tested the method on palladium-catalysed C-N cross-coupling reactions, making amines that are common in biologically active molecules. The reactions couple aryl halides to nitrogen nucleophiles and form byproducts – inorganic salts – that are insoluble in the solvents used.
As a result, says Noël, they were able to obtain diarylamine products with reaction times ranging from 20 seconds to 10 minutes. At very short residence times (time in the reactor under reaction conditions) they observed a significantly higher rate for the reaction in flow compared to the equivalent batch experiments. With high conversions in short reaction times, they were able to reduce the catalyst loading in flow to just 0.1 mol per cent. ‘Extremely low catalyst loadings such as these are of particular interest to the pharmaceutical industry,’ says Noël.
Noël believes that in the future microfluidics will be used to construct increasingly complex molecules. Different devices will automate and integrate many synthetic steps that are currently performed using the more traditional and time-consuming batch-based practices.
Oliver Kappe, from the Christian Doppler Laboratory for Microwave Chemistry, Institute of Chemistry, Karl-Franzens-University Graz says: ‘Jensen and Buchwald clearly demonstrate that immersing a flow device into an ultrasound bath can prevent clogging problems that unfortunately are all too familiar to the flow/microreactor community.’
Direct Fluorination and Microreactor Technology
Elemental fluorine has long been considered to be too reactive and uncontrollable for use as a reagent in organic synthesis and this perception still predominates. Prof. Poliakoff’s comments on the popular Periodic Table video series (www.PeriodicVideos.com), ‘It was much more exciting than I thought …you see the flames,’ and general comments in standard advanced organic chemistry textbooks (J. March, Advanced Organic Chemistry, ‘Direct fluorination of aromatic rings with F2 is not feasible at room temperature because of the extreme reactivity of F2….not yet of preparative significance) are typical.
Despite this background, research into the use of elemental fluorine for organic synthesis at Durham has overcome the many problems of using fluorine gas for the safe synthesis of fine chemicals, in particular, by use of dilute fluorine gas in nitrogen, appropriate solvent choice (high dielectric constant media such as formic acid, sulfuric acid or acetonitrile), reactor vessel design, gas flow regulator systems and stainless steel/monel fluorine gas handling lines have developed over the years to allow selective direct fluorination of a range of aliphatic, dicarbonyl, aromatic, heteroaromatic, heterocyclic, steroid and carbohydrate derivatives to be established and the mechanism (regiochemistry, stereochemistry, selectivity, etc.) of these processes to be assessed. Indeed, direct fluorination of aromatic rings is feasible at room temperature ! Research expanding the use of fluorine gas continues to develop new selective fluorination methodology for the synthesis of a range of aromatic, heterocylic and aliphatic systems.2,3

In particular, a process for the synthesis of a fluoroketoester first carried out in Durham was developed by our industrial collaborators, F2 Chemicals Ltd (UK), for the Pfizer company and forms a key starting material in the multi step synthesis of the widely used anti-fungal agent V-Fend (Voriconazole) throughout the clinical trial, launch and commercialization periods. In the period from January 2008 to March 2011 approximately 17 tonnes of the fluoroketoester were manufactured for Pfizer by F2 Chemicals Ltd. Global sales of V-Fend in the 2008-2010 period total $2.4 billion (Pfizer annual financial reports) and in 2010 was 17th position in Pfizer’s best selling products and it is one of the global top 100 best selling pharmaceutical products.

Further reaction control in selective fluorination reactions was achieved by the design, fabrication and commissioning of single and multi-channel continuous flow reactor systems, establishing the use of convenient, inexpensive flow reactors for gas – liquid processes using flow regimes in the laboratory. Techniques for the supply of individual gas and liquid reagents from single sources to a parallel array of many flow channels at the same flow rate and pressure whilst maintaining laminar flow within the reactor channels and telescoped gas – liquid / liquid – liquid processes involving fluorination and ring formation in one continuous flow process have been developed.

References
- Roughley, S. D.; Jordan, A. M. (2011). “The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates”. J. Med. Chem. 54: 3451.
- Dach, R.; Song, J. J.; Roschangar, F.; Samstag, W.; Senanayake, C. H. (2012). “The eight criteria defining a good chemical manufacturing process”. Org. Process Res. Dev. 16: 1697.
- Trost, B. M. (1991). “The atom economy – a search for synthetic efficiency”. Science 254: 1471.
- Van Aken, K.; Strekowski, L.; Patiny, L. (2006). “EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters”. Beilstein J. Org. Chem. 2 (No. 3).
- Faucher, A-M.; Bailey, M. D.; Beaulieu, P. L.; Brochu, C.; Duceppe, J-S.; Ferland, J-M.; Ghiro, E.; Gorys, V.; Halmos, T.; Kawai, S. H.; Poirier, M.; Simoneau, B.; Tsantrizos, Y. S.; Llinas-Brunet, M. (2004). “Synthesis of BILN 2061, an HCV NS3 protease inhibitor with proven antiviral effect in humans”. Org. Lett. 6: 2901.
- Yee, N. K.; Farina, V.; Houpis, I. N.; Haddad, N.; Frutos, R. P.; Gallou, F.; Wang, X-J.; Wei, X.; Simpson, R. D.; Feng, X.; Fuchs, V.; Xu, Y.; Tan, J.; Zhang, L.; Xu, J.; Smith-Keenan, L. L.; Vitous, J.; Ridges, M. D.; Spinelli, E. M.; Johnson, M. (2006). “Efficient large-scale synthesis of BILN 2061, a potent HCV protease inhibitor, by a convergent approach based on ring-closing metathesis”. J. Org. Chem. 71: 7133.
- Zeng, X.; Wei, X.; Farina, V.; Napolitano, E.; Xu, Y.; Zhang, L.; Haddad, N.; Yee, N. K.; Grinberg, N.; Shen, S.; Senanayake, C. H. (2006). “Epimerization reaction of a substituted vinylcyclopropane catalyzed by ruthenium carbenes: mechanistic analysis”. J. Org. Chem. 71: 8864.
- Grela, K.; Harutyunyan, S.; Michrowska, A. (2002). “A highly efficient ruthenium catalyst for metathesis reactions”. Angew. Chem. Int. Ed. 41: 4038.
- Wei, X.; Shu, C.; Haddad, N.; Zeng, X.; Patel, N. D.; Tan, Z.; Liu, J.; Lee, H.; Shen, S.; Campbell, S.; Varsolona, R. J.; Busacca, C. A.; Hossain, A.; Yee, N. K.; Senanayake, C. H. (2013). “A highly convergent and efficient synthesis of a macrocyclic hepatitis C virus protease inhibitor BI 201302”. Org. Lett. 15: 1016.
- Bornscheuer, U. T.; Huisman, G. W.; Kazlauskas, R. J.; Lutz, S.; Moore, J. C.; Robins, K. (2012). “Engineering the third wave of biocatalysis”. Nature 485: 185.
- Savile, C. K.; Janey, J. M.; Mundorff, E. C.; Moore, J. C.; Tam, S.; Jarvis, W. R.; Colback, J. C.; Krebber, A.; Fleitz, F. J.; Brands, J.; Devine, P. N.; Huisman, G. W.; Hughes, G. J. (2010). “Biocatalytic asymmetric synthesis of chiral amines applied to sitagliptin manufacture”. Science 329: 305.
- Desai, A. A. (2011). “Sitagliptin manufacture: a compelling tale of green chemistry, process intensification, and industrial asymmetric catalysis”. Angew. Chem. Int. Ed. 50: 1974.
- Busacca, C. A.; Fandrick, D. R.; Song, J. J.; Sananayake, C. H. (2011). “The growing impact of catalysis in the pharmaceutical industry”. Adv. Synth. Catal. 353: 1825.
- Johnson, M. D.; May, S. A.; Calvin, J. R.; Remacle, J.; Stout, J. R.; Dieroad, W. D.; Zaborenko, N.; Haeberle, B. D.; Sun, W-M.; Miller, M. T.; Brannan, J. (2012). “Development and scale-up of a continuous, high-pressure, asymmetric hydrogenation reaction, workup, and isolation”. Org. Process Res. Rev. 16: 1017.
249,133 total views, 4 views today