Polymorphism, Case studies

Case 1
QbD based Crystallization Process Development for the Polymorphic Drug Tolbutamide
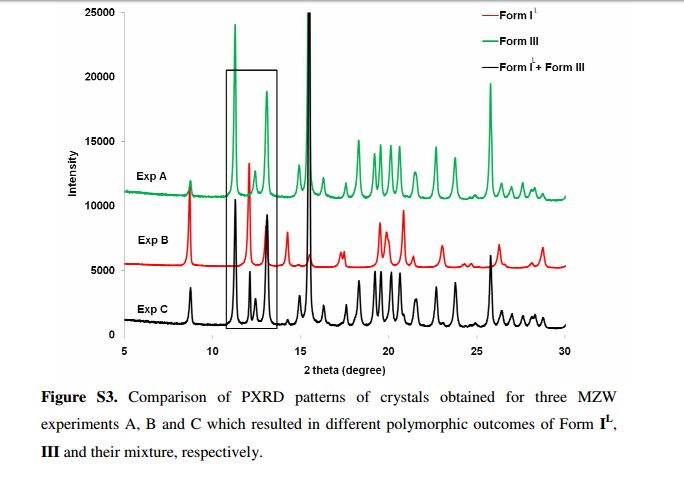
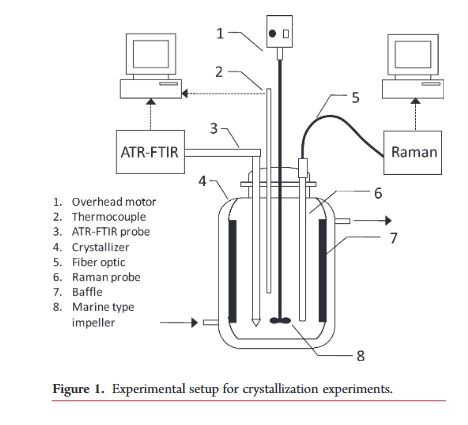
The thermodynamically stable Form II of the antidiabetic drug tolbutamide exhibits a thin fiber needle shape which renders it intractable for isolation and downstream processing. This work implements two in situ process analytical technology (PAT) methods, namely, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) with orthogonal partial least-squares-principal component analysis (OPLS-PCA) for monitoring solute concentration, and Raman spectroscopy with dynamic PCA-based multivariate statistical process monitoring (MSPM) for detection of solid form purity, to derive the robust design space for cooling crystallization of the desired Form IL.

The thermodynamically stable Form II of the antidiabetic drug tolbutamide exhibits a thin fiber needle shape which renders it intractable for isolation and downstream processing. This work implements two in situ process analytical technology (PAT) methods, namely, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) with orthogonal partial least-squares-principal component analysis (OPLS-PCA) for monitoring solute concentration, and Raman spectroscopy with dynamic PCA based multivariate statistical process monitoring (MSPM) for detection of solid form purity, to derive the robust design space for cooling crystallization of the desired Form IL.
WILL BE UPDATED WITH MORE, WATCH OUT

/////////////
25,707 total views, 1 views today