GW Pharmaceuticals has been boosted by the news that the US Food and Drug Administration has granted fast-track designation to the UK firm’s investigational cannabidiol Epidiolex, in the treatment of Dravet syndrome.
Read more at: http://www.pharmatimes.com/Article/14-06-06/GW_Pharma_s_Epidiolex_on_fast_track_in_USA.aspx#ixzz342QAmTzl
old aticle

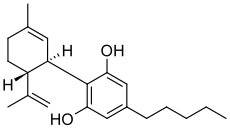 Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -
Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -
LONDON, Nov. 15, 2013
GW Pharmaceuticals plc (AIM: GWP, Nasdaq: GWPH, “GW”) announced today that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation for Epidiolex(R), our product candidate that contains plant-derived Cannabidiol (CBD) as its active ingredient, for use in treating children with Dravet syndrome, a rare and severe form of infantile-onset, genetic, drug-resistant epilepsy syndrome. Epidiolex is an oral liquid formulation of a highly purified extract of CBD, a non-psychoactive molecule from the cannabis plant. Following receipt of this orphan designation, GW anticipates holding a pre-IND meeting with the FDA in the near future to discuss a development plan for Epidiolex in Dravet syndrome.
Dravet syndrome is a rare pediatric epilepsy syndrome with a distinctive but complex electroclinical presentation. Onset of Dravet syndrome occurs during the first year of life with clonic and tonic-clonic seizures in previously healthy and developmentally normal infants. Prognosis is poor and patients typically develop intellectual disability and life-long ongoing seizures. There are approximately 5,440 patients with Dravet in the United States and an estimated 6,710 Dravet patients in Europe. These figures may be an underestimate as this syndrome is reportedly underdiagnosed.
In addition to GW’s clinical development program for Epidiolex in Dravet syndrome, which is expected to commence in 2014, GW has also made arrangements to enable independent U.S. pediatric epilepsy specialists to treat high need pediatric epilepsy cases with Epidiolex immediately. To date in 2013, a total of seven “expanded access” INDs have been granted by the FDA to U.S. clinicians to allow treatment with Epidiolex of approximately 125 children with epilepsy. These children suffer from Dravet syndrome, Lennox-Gastaut syndrome, and other pediatric epilepsy syndromes. GW is aware of further interest from additional U.S. and ex-U.S. physicians to host similar INDs for Epidiolex. GW expects data generated under these INDs to provide useful observational data during 2014 on the effect of Epidiolex in the treatment of a range of pediatric epilepsy syndromes.
“I, together with many colleagues in the U.S. who specialize in the treatment of childhood epilepsy, very much welcome the opportunity to investigate Epidiolex in the treatment of Dravet syndrome. The FDA’s timely approval of the orphan drug designation for Epidiolex in Dravet syndrome is a key milestone that comes after many years of reported clinical cases that suggest encouraging evidence of efficacy for CBD in this intractable condition,” stated Dr. Orrin Devinsky, Professor of Neurology, Neurosurgery and Psychiatry in New York City. “With GW now making plans to advance Epidiolex through an FDA development program, we have the prospect for the first time of fully understanding the science of CBD in epilepsy with a view to making an appropriately tested and approved prescription medicine available in the future for children who suffer from this debilitating disease.”
“GW is proud to be at the forefront of this important new program to treat children with Dravet Syndrome and potentially other forms of intractable childhood epilepsy. For families in these circumstances, their lives are significantly impacted by constant and often times very severe seizures in children where all options to control these seizures have been exhausted,” stated Dr. Stephen Wright, GW’s R&D Director. “GW intends to advance a full clinical development program for Epidiolex in Dravet syndrome as quickly as possible, whilst at the same time helping families in the short term through supporting physician-led INDs to treat intractable cases. Through its efforts, GW aims to provide the necessary evidence to confirm the promise of CBD in epilepsy and ultimately enabling children to have access to an FDA-approved prescription CBD medicine.”
“This orphan program for Epidiolex in childhood epilepsy is an important corporate strategic priority for GW. Following receipt of today’s orphan designation, GW now intends to commence discussions with the FDA regarding the U.S. regulatory pathway for Epidiolex,” stated Justin Gover, GW’s Chief Executive Officer. “GW intends to pursue this development in-house and retains full commercial rights to Epidiolex.”
About Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition — generally a disease or condition that affects fewer than 200,000 individuals in the U.S. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication.
About GW Pharmaceuticals plc
Founded in 1998, GW is a biopharmaceutical company focused on discovering, developing and commercializing novel therapeutics from its proprietary cannabinoid product platform in a broad range of disease areas. GW commercialized the world’s first plant-derived cannabinoid prescription drug, Sativex(R), which is approved for the treatment of spasticity due to multiple sclerosis in 22 countries. Sativex is also in Phase 3 clinical development as a potential treatment of pain in people with advanced cancer. This Phase 3 program is intended to support the submission of a New Drug Application for Sativex in cancer pain with the U.S. Food and Drug Administration and in other markets around the world. GW has established a world leading position in the development of plant-derived cannabinoid therapeutics and has a deep pipeline of additional clinical-stage cannabinoid product candidates targeting epilepsy (including an orphan pediatric epilepsy program), Type 2 diabetes, ulcerative colitis, glioma and schizophrenia. For further information, please visithttp://www.gwpharm.com.
Cannabidiol (CBD) is one of at least 85 cannabinoids found in cannabis.It is a major constituent of the plant, second to tetrahydrocannabinol (THC), and represents up to 40% in its extracts. Compared with THC, cannabidiol is not psychoactive in healthy individuals, and is considered to have a wider scope of medical applications than THC, including to epilepsy, multiple sclerosis spasms,anxiety disorders, bipolar disorder,schizophrenia,nausea, convulsion and inflammation, as well as inhibiting cancer cell growth. There is some preclinical evidence from studies in animals that suggests CBD may modestly reduce the clearance of THC from the body by interfering with its metabolism.Cannabidiol has displayed sedative effects in animal tests. Other research indicates that CBD increases alertness. CBD has been shown to reduce growth of aggressive human breast cancer cells in vitro, and to reduce their invasiveness.















 Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -
Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -







 Microgel-based thermoresponsive membranes for water filtration
Microgel-based thermoresponsive membranes for water filtration