| Synthesis a) |

 |
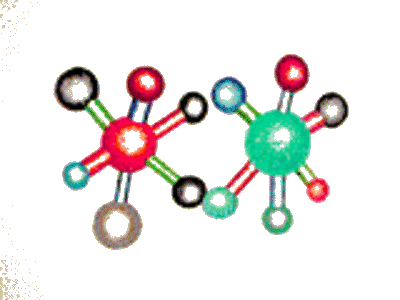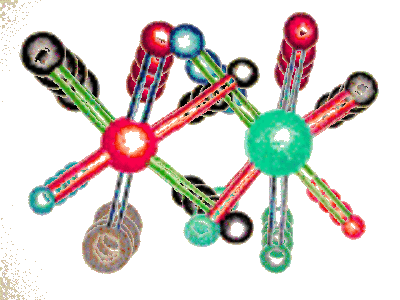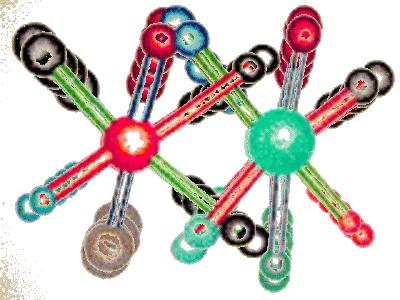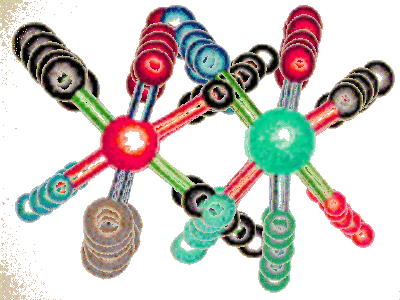
Synthetic route
The reaction of 2- (1-imidazolyl) acetonitrile (I) with CS2 and KOH in DMF gives the dithiolate (II), which is then cyclized with 1- (2-chlorophenyl) -1,2-di (methanesulfonyloxy) ethane . (III) A column chromatography over silicagel allows the separation of the (E) -.? and (Z) -isomers (1-5)
Description Crystals, mp 141-5 Manufacturer Nihon Nohyaku Co., Ltd. (Japan) and Tsumura Juntendo (Japan).
References 1. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). Antimycotic agent and fungicidal agent. US 4738976. 2. Seo, A ., Sugano, H., Hasegawa, C., Ikeda, K., Munechica, Y., Konoe, T., Konaka, M. (Nihon Nohyaku Co., Ltd.). Antifungal agent. JP 87093227. 3. Seo , A., Sugano, H., Hasegawa, C., Ikeda, K., Nishimura, A., Miyashiro, Y. (Nihon Nohyaku Co., Ltd.). Non-medicinal bactericidal agents and method for their preparation. JP 87093204. 4. Seo, A., Sugano, H., Hasegawa, C., Miyashiro, Y., Nishimura, A., Ikeda, K. (Nihon Nohyaku Co., Ltd.). Ketene S, S-acetals. JP 85218387. 5. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). A novel ketene S, S-acetal deriv., a process for manufacturing thereof and a method for curing mycosis by administering it. EP 218736.
References
1. Oka, H., et al., 1992. Therapeutic efficacy of latoconazole in formulations of clinical use on experimental dermatophytosis in guinea pigs. Arzneimittel-Forschung. 42(3): 345-9. PMID: 1497697
2. Niwano, Y., et al., 1994. Therapeutic efficacy of lanoconazole, a new imidazole antimycotic agent, for experimental cutaneous candidiasis in guinea pigs. Antimicrobial agents and chemotherapy. 38(9): 2204-6. PMID: 7811048
3 http://aac.asm.org/content/38/9/2204.full.pdf
References 1. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). Antimycotic agent and fungicidal agent. US 4738976. 2. Seo, A ., Sugano, H., Hasegawa, C., Ikeda, K., Munechica, Y., Konoe, T., Konaka, M. (Nihon Nohyaku Co., Ltd.). Antifungal agent. JP 87093227. 3. Seo , A., Sugano, H., Hasegawa, C., Ikeda, K., Nishimura, A., Miyashiro, Y. (Nihon Nohyaku Co., Ltd.). Non-medicinal bactericidal agents and method for their preparation. JP 87093204. 4. Seo, A., Sugano, H., Hasegawa, C., Miyashiro, Y., Nishimura, A., Ikeda, K. (Nihon Nohyaku Co., Ltd.). Ketene S, S-acetals. JP 85218387. 5. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). A novel ketene S, S-acetal deriv., a process for manufacturing thereof and a method for curing mycosis by administering it. EP 218736.
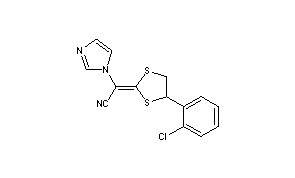
Title: Lanoconazole
CAS Registry Number: 101530-10-3
CAS Name: (E)-(±)-a-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene]-1H-imidazole-1-acetonitrile
Additional Names: latoconazole
Manufacturers’ Codes: TJN-318; NND-318
Trademarks: Astat (Nihon Nohyaku)
Molecular Formula: C14H10ClN3S2
Molecular Weight: 319.83
Percent Composition: C 52.57%, H 3.15%, Cl 11.08%, N 13.14%, S 20.05%
Literature References: Prepn: A. Soe et al., JP Kokai 85 218387; idem et al., US 4636519 (1985, 1987 both to Nihon Nohyaku).In vivo antifungal activity: H. Oka et al., Arzneim.-Forsch. 42, 345 (1992); Y. Niwano et al., Antimicrob. Agents Chemother. 38,2204 (1994). Toxicity study: P. L. Munt et al., Oyo Yakuri 43, 195 (1992).
Properties: Light yellow crystals, mp 141.5°. LD50 in male, female mice, rats (mg/kg): 3224, 2715, 993, 652 orally; 2158, 1743, 1655, 2596 i.p.; >5000 both species s.c. LD50 dermally in rats: >5000 mg/kg (Munt).
Melting point: mp 141.5°
Toxicity data: LD50 in male, female mice, rats (mg/kg): 3224, 2715, 993, 652 orally; 2158, 1743, 1655, 2596 i.p.; >5000 both species s.c.; LD50 dermally in rats: >5000 mg/kg (Munt)
Therap-Cat: Antifungal.
Keywords: Antifungal (Synthetic); Imidazoles.




























































 The National Chemical Laboratory is located in the state of Maharashtra in India. Maharashtra state is the largest contributor to India’s GDP. The National Chemical Laboratory is located in Pune city, and is the cultural capital of Maharashtra. Pune city is second only to Mumbai (the business capital of India) in size and industrial strength. Pune points of interest include: The tourist places in Pune include: Lal Deval Synagogue, Bund Garden, Osho Ashram, Shindyanchi Chhatri and Pataleshwar Cave Temple.
The National Chemical Laboratory is located in the state of Maharashtra in India. Maharashtra state is the largest contributor to India’s GDP. The National Chemical Laboratory is located in Pune city, and is the cultural capital of Maharashtra. Pune city is second only to Mumbai (the business capital of India) in size and industrial strength. Pune points of interest include: The tourist places in Pune include: Lal Deval Synagogue, Bund Garden, Osho Ashram, Shindyanchi Chhatri and Pataleshwar Cave Temple.



