tert-Butyl 3a,4,7,7a-Tetrahydro-1H-isoindole-2(3H)-carboxylate
tert-Butyl 3a,4,7,7a-Tetrahydro-1H-isoindole-2(3H)-carboxylate
tert-Butyl 3a,4,7,7a-Tetrahydro-1H-isoindole-2(3H)-carboxylate
tert-Butyl 3a,4,7,7a-Tetrahydro-1H-isoindole-2(3H)-carboxylate
2,2′-(1-(tert-Butoxycarbonyl)pyrrolidine-3,4-diyl)diacetic Acid
2,2′-(1-(tert-Butoxycarbonyl)pyrrolidine-3,4-diyl)diacetic Acid
Dimethyl 4,4′-(Benzylazanediyl)(2E,2′E)-bis(but-2-enoate)
IR (CHCl3): ν = 758, 1215, 1278, 1437, 1660, 1720, 2806, 2953, 3020, 3421 cm–1;
13C NMR (CDCl3, 100 MHz) δ: 51.53, 53.42, 58.37, 122.66, 127.28, 128.41, 128.55, 128.76, 138.24, 145.84, 166.58;
1H NMR (CDCl3, 400 MHz) δ: 3.23 (dd, J1 = 1.6 Hz, J2 = 6.0 Hz, 4H), 3.62 (s, 2H), 3.75 (s, 6H), 6.07 (dt, J1 = 1.6 Hz, J2 = 16.0 Hz, 2H), 6.97 (dt, J1 = 6.0 Hz, J2 = 16.0 Hz, 2H), 7.25–7.34 (m, 5H-merged with CDCl3 proton);
TOFMS: [C17H21NO4 + H+]: calculated 304.1543, found 304.1703(100%).
UPLC conditions were as follows for compound 11; Acquity Waters, column: BEH C18 (2.1 mm X 100 mm) 1.7 µm with mobile phases A (0.05% TFA in water) and B (acetonitrile). Detection was at 220 nm, flow was set at 0.4 mL/min, and the temperature was 30 °C (Run time: 9 min). Gradient: 0 min, A = 90%, B = 10%; 0.5 min, A = 90%, B = 10%; 6.0 min, A = 0%, B = 100%; 7.5 min, A = 0%, B = 100%; 7.6 min, A = 90%, B = 10%; 9.0 min, A = 90%, B = 10%.
Dimethyl 4,4′-(Benzylazanediyl)(2E,2′E)-bis(but-2-enoate) (11)
1-Bromo-4-fluoro-2-((2-iodobenzyl)oxy)benzene
CAS 1161931-51-6
Mp 89.8–92.3 °C.
IR (neat, ATR): 3072 (w), 1482 (s), 1451 (s), 1294 (s), 1294 (s) cm–1.
1H NMR (399 MHz, DMSO-d6) δ 5.12 (s, 2H), 6.81 (td, J = 8.49, 2.77 Hz, 1H), 7.14 (td, J = 7.64, 1.65 Hz, 1H), 7.18 (dd, J = 10.90, 2.82 Hz, 1H), 7.46 (td, J = 7.52, 0.92 Hz, 1H), 7.60 (dd, J = 7.64, 1.41 Hz, 1H), 7.62 (dd, J = 8.66, 6.23 Hz, 1H), 7.92 (dd, J = 7.83, 0.83 Hz, 1H).
13C NMR (100 MHz, DMSO-d6) δ 74.5, 99.2, 102.4 (d, J = 27.1 Hz), 105.8 (d, J = 3.4 Hz), 108.9 (d, J = 22.5 Hz), 128.5, 129.8, 130.3, 133.6 (d, J = 9.9 Hz), 138.0, 139.2, 155.4 (d, J = 10.7 Hz), 162.2 (d, J = 244.3 Hz).
GCMS: m/z [M]+ calcd for C13H9BrFIO: 405.88600; found: 405.88620.
1H AND 13C NMR PREDICT
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

Hana and co-workers ( Synlett 2010, 18, 2759−2764) from Genentech have developed a single-step procedure for conversion of 2-nitro aromatic amines to benzimidazoles. Addition of ammonium chloride proved necessary as Fe powder and formic acid alone was ineffective for nitro reduction. These conditions were compatible with a variety of functional groups on the aromatic, including boronate esters. The methodology was also extended to nitro aminopyridines but failed to deliver the desired product with isoxazole or pyrazole reactants.
*Discovery Chemistry, Genentech, Inc., 1 DNA Way, South San Francisco, CA 94080, USA, Email: hanan.emily gene.com
gene.com
E. J. Hanan, B. K. Chan, A. A. Estrada, D. G. Shore, J. P. Lyssikatos, Synlett, 2010, 2759-2764.

see article for more reactions
Abstract
A one-pot procedure for the conversion of aromatic and heteroaromatic 2-nitroamines into bicyclic 2H-benzimidazoles employs formic acid, iron powder, and NH4Cl as additive to reduce the nitro group and effect the imidazole cyclization with high-yielding conversions generally within one to two hours. The compatibility with a wide range of functional groups demonstrates the general utility of this procedure.

see article for more examples
//////////One-Pot, Reductive Cyclisations, Nitroanilines, Imidazoles
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
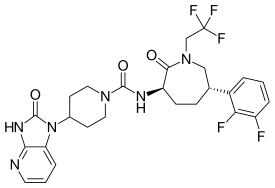
Telcagepant, MK-0974
Migraine is a neurovascular disorder characterized by severe, debilitating, and throbbing unilateral headache. Though a leading cause of disability, it is a highly prevalent disease with a clear unmet medical need. With the significant progress achieved in the field of pathophysiology in the past decades, to date, it is well recognized that the neuropeptide calcitonin gene-related peptide (CGRP), which is expressed mainly in the central and peripheral nervous system, plays a crucial role in migraine. Antagonism of CGRP receptors, as a potential new therapy for the treatment of migraine, could offer the advantage of avoiding the cardiovascular liabilities associated with other existing antimigraine therapies.
Telcagepant (INN) (code name MK-0974) is a calcitonin gene-related peptide receptor antagonist which was an investigational drug for the acute treatment and prevention of migraine, developed by Merck & Co. In the acute treatment of migraine, it was found to have equal potency to rizatriptan[1] and zolmitriptan[2] in two Phase III clinical trials. The company has now terminated development of the drug.
The calcitonin gene-related peptide (CGRP) is a strong vasodilator primarily found in nervous tissue. Since vasodilation in the brain is thought to be involved in the development of migraine and CGRP levels are increased during migraine attacks, this peptide may be an important target for potential new antimigraine drugs.
Telcagepant acts as a calcitonin gene-related peptide receptor (CRLR) antagonist and blocks this peptide. It is believed to constrict dilated blood vessels within the brain.[3]
A Phase IIa clinical trial studying telcagepant for the prophylaxis of episodic migraine was stopped on March 26, 2009 after the “identification of two patients with significant elevations in serum transaminases”.[4] A memo to study locations stated that telcagepant had preliminarily been reported to increase the hepatic liver enzyme alanine transaminase (ALT) levels in “11 out of 660 randomized (double-blinded) study participants.” All study participants were told to stop taking the medication.[5]
On July 29, 2011, it was reported that Merck & Co. were discontinuing the clinical development program for telcagepant. According to Merck, “[t]he decision is based on an assessment of data across the clinical program, including findings from a recently completed six-month Phase III study”.[6]
CLIP



CLIP
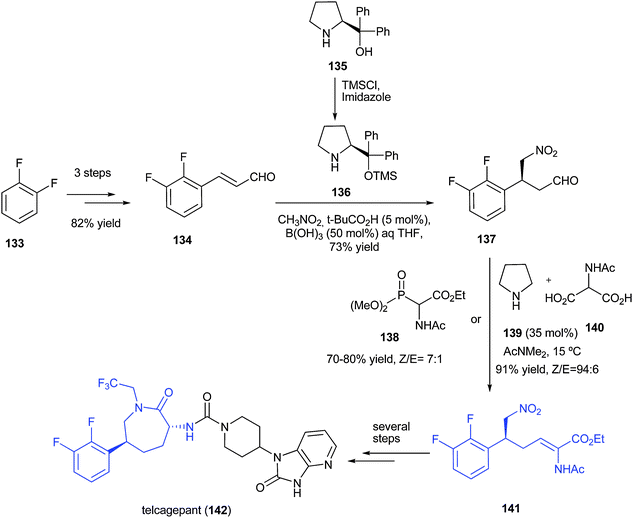
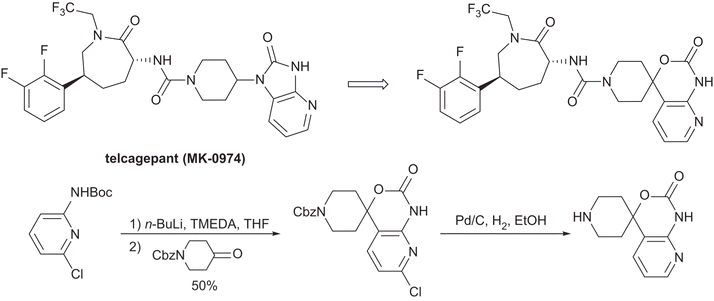
CLIP
http://pubs.acs.org/doi/abs/10.1021/jo101704b




 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 5–8 hours |
| Identifiers | |
| CAS Number | 781649-09-0 |
| PubChem (CID) | 11319053 |
| IUPHAR/BPS | 703 |
| ChemSpider | 9494017 |
| UNII | D42O649ALL |
| KEGG | D09391 |
| ChEMBL | CHEMBL236593 |
| Chemical and physical data | |
| Formula | C26H27F5N6O3 |
| Molar mass | 566.5283 g/mol |
| 3D model (Jmol) | Interactive image |
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|---|---|---|
| US8394767 | Methods of treating cancer using the calcitonin-gene related peptide (â??CGRPâ??) receptor antagonist CGRP8-37 | 2011-01-10 | 2013-03-12 |
| US8080544 | PRODRUGS OF CGRP RECEPTOR ANTAGONISTS | 2010-11-25 | 2011-12-20 |
| US7893052 | CGRP RECEPTOR ANTAGONISTS | 2010-11-25 | 2011-02-22 |
| US2010286122 | CGRP Antagonist Salt | 2010-11-11 | |
| US7829699 | Process for the Preparation of Cgrp Antagonist | 2009-11-12 | 2010-11-09 |
| US7772224 | CGRP RECEPTOR ANTAGONISTS | 2009-07-30 | 2010-08-10 |
| US7745427 | Cgrp Receptor Antagonists | 2008-04-17 | 2010-06-29 |
| US7718796 | Process for the preparation of Caprolactam Cgrp Antagonist | 2009-05-14 | 2010-05-18 |
| US2010009967 | SOLID DOSAGE FORMULATIONS OF TELCAGEPANT POTASSIUM | 2010-01-14 | |
| US2009176986 | Process for the Preparation of Pyridine Heterocycle Cgrp Antagonist Intermediate | 2009-07-09 |
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
C1CC(C(=O)N(CC1C2=C(C(=CC=C2)F)F)CC(F)(F)F)NC(=O)N3CCC(CC3)N4C5=C(NC4=O)N=CC=C5
A convergent, robust, and concise synthesis of a developmental CCR1 antagonist is described using continuous flow technology. In the first approach, following an expeditious SNAr sequence for cyclopropane introduction, a safe, continuous flow Curtius rearrangement was developed for the synthesis of a p-methoxybenzyl (PMB) carbamate. Based on kinetic studies, a highly efficient and green process comprising three chemical transformations (azide formation, rearrangement, and isocyanate trapping) was developed with a relatively short residence time and high material throughput (0.8 kg h−1, complete E-factor = ∼9) and was successfully executed on 40 kg scale. Moreover, mechanistic studies enabled the execution of a semi-continuous, tandem Curtius rearrangement and acid–isocyanate coupling to directly afford the final drug candidate in a single, protecting group-free operation. The resulting API synthesis is further determined to be extremely green (RPG = 166%) relative to the industrial average for molecules of similar complexity.
DOI: 10.1039/C6GC03123D
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
m.p. = 140-144 °C;
1H NMR (400 MHz, CDCl3) δ 9.76 (s, 1H), 9.43 (s, 1H), 8.95 (s, 1H), 8.70 (s, 1H), 8.68 (d, J = 5.2 Hz, 1H), 7.93 (s, J1 = 8.8 Hz, J2 = 4.7 Hz, 1H), 7.82 (s, 1H), 7.54 (d, J = 4.1 Hz, 1H), 7.49 (t, J = 8.7 Hz, 1H), 3.29 (s, 3H), 1.61 (bs, 4H);
13C NMR (100 MHz, CDCl3) δ 166.1, 162.7, 160.3, 158.4, 156.9, 150.6, 139.2, 138.2, 135.8, 135.6, 125.4 (d, JC-F = 8.8 Hz), 123.3, 121.9, 117.2 (d, JC-F = 23.1 Hz), 116.4, 40.2, 34.9, 20.9;
HRMS: calcd for C22H19FN5O3S [M + H+ ]: 452.1187. Found: 452.1189.
//////////BI-638683, BI 638683, CCR1 antagonist, 295298-26-8, US2012270870, Boehringer Ingelheim Pharmaceuticals, phase 1
CS(=O)(=O)c1nccc(c1)C2(CC2)NC(=O)c5cncc3c5cnn3c4ccc(F)cc4
| Molecular Formula: | C22H18FN5O3S |
|---|---|
| Molecular Weight: | 451.476 g/mol |
cas 295298-26-8
maybe BI-638683, not sure
In September 2010, a randomized, double-blind, placebo-controlled, phase I study (NCT01195688; 1279.1; 2010-021187-15) was initiated in healthy male volunteers (expected n = 64) in Germany, to assess the safety, pharmacokinetics and pharmacodynamics of BI-638683. The study was completed in December 2010 . In June 2014, data were presented at the EULAR 2014 Annual Meeting in Paris, France. A dose of 75-mg showed maximal inhibition of mRNA expression of the four-CC chemokine receptor type-I dependent marker genes. chemokine ligand -2 and Peroxisome proliferator-activated receptor gamma-mRNAs by doses of 300 mg and higher, and for Ras-related protein rab-7b mRNA by doses of 500 mg and higher
Boehringer Ingelheim was developing BI-638683, a CCR1 antagonist, for the potential oral treatment of rheumatoid arthritis. A phase I trial was completed in December 2010 . Phase I data was presented in June 2014
| Inventors | Brian Nicholas Cook, Daniel Kuzmich, Can Mao, Hossein Razavi |
| Applicant | Boehringer Ingelheim International Gmbh |
Chemotactic Cytokine Receptor 1 (CCRl) belongs to a large family (>20) of chemotactic cytokine (chemokine) receptors that interact with specific chemokines (>50) to mediate leukocyte trafficking, granule exocytosis, gene transcription, mitogenic effects and apoptosis. Chemokines are best known for their ability to mediate basal and inflammatory leukocyte trafficking. The binding of at least three chemokines (MIP-1 alpha/CCL3, MCP3/CCL7 and RANTES/CCL5) to CCRl is responsible for the trafficking of monocytes, macrophages and THl cells to inflamed tissues of rheumatoid arthritis (RA) and multiple sclerosis (MS) patients (Trebst et al. (2001) American J of Pathology 159 p. 1701). Macrophage inflammatory protein 1 alpha (MIP-1 alpha), macrophage chemoattractant protein 3 (MCP-3) and regulated on activation, normal T-cell expressed and secreted (RANTES) are all found in the CNS of MS patients, while MIP-1 alpha and RANTES are found in the CNS in the experimental autoimmune encephalomyelitis (EAE) model of MS (Review: Gerard
and Rollins (2001) Nature Immunology). Macrophages and Thl cells in the inflamed synovia of RA patients are major producers of MIP-1 alpha and RANTES, which continuously recruit leukocytes to the synovial tissues of RA patients to propagate chronic inflammation (Volin et al. (1998) Clin. Immunol. Immunopathology; Koch et al. (1994) J. Clin. Investigation; Conlon et al. (1995) Eur. J. Immunology). Antagonizing the interactions between CCR1 and its chemokine ligands is hypothesized to block chemotaxis of monocytes, macrophages and Thl cells to inflamed tissues and thereby ameliorate the chronic inflammation associated with autoimmune diseases such as RA and MS.
Evidence for the role of CCR1 in the development and progression of chronic inflammation associated with experimental autoimmune encephalitis (EAE), a model of multiple sclerosis, is based on both genetic deletion and small molecule antagonists of CCR1. CCR1 deficient mice were shown to exhibit reduced susceptibility (55% vs. 100%) and reduced severity (1.2 vs. 2.5) of active EAE (Rottman et al. (2000) Eur. J. Immunology). Furthermore, administration of small molecule antagonist of CCR1, with moderate affinity (K; = 120 nM) for rat CCR1, was shown to delay the onset and reduce the severity of EAE when administered intravenously (Liang et al. (2000) /. Biol. Chemistry). Treatment of mice with antibodies specific for the CCR1 ligand MIP- 1 alpha have also been shown to be effective in preventing development of acute and relapsing EAE by reducing the numbers of T cells and macrophages recruited to the CNS (Karpus et al. (1995) /. Immunology; Karpus and Kennedy (1997) /. Leukocyte Biology). Thus, at least one CCR1 ligand has been demonstrated to recruit leukocytes to the CNS and propagate chronic inflammation in EAE, providing further in vivo validation for the role of CCR1 in EAE and MS.
In vivo validation of CCR1 in the development and propagation of chronic inflammation associated with RA is also significant. For example, administration of a CCR1 antagonist in the collagen induced arthritis model (CIA) in DBA/1 mice has been shown to be effective in reducing synovial inflammation and joint destruction (Plater-Zyberk et al. (1997) Immunology Letters). Another publication described potent antagonists of murine CCR1 that reduced severity (58%) in LPS-accelerated collagen-induced arthritis (CIA), when administered orally {Biorganic and Medicinal Chemistry Letters 15, 2005, 5160-5164). Published results from a Phase lb clinical trial with an oral CCRl antagonist demonstrated a trend toward clinical improvement in the absence of adverse side effects (Haringman et al. (2003) Ann. Rheum. Dis.). One third of the patients achieved a 20% improvement in rheumatoid arthritis signs and symptoms (ACR20) on day 18 and CCRl positive cells were reduced by 70% in the synovia of the treated patients, with significant reduction in specific cell types including 50% reduction in CD4+ T cells, 50% reduction in CD8+ T cells and 34% reduction in macrophages.
Studies such as those cited above support a role for CCRl in MS and RA and provide a therapeutic rationale for the development of CCRl antagonists.
http://www.fda.moph.go.th/eng/index.stm
www.jpsbr.org/index_htm_files/JPSBR14RV4029.pdf
Apr 13, 2014 – Thailand has its own drug registration format and also follows. ASEAN CTD. … Transparency in the regulatory authorities of member countries.
The Thai FDA (TFDA), one of several agencies under the Ministry of Public Health (MPH), is the regulatory body administering drugs in Thailand. The Drug Control Division of the TFDA is responsible for registration, licensing, surveillance, inspection and adverse event monitoring for all pharmaceuticals and pharmaceutical companies in Thailand. Foreign pharma companies dominate the Thai drug market. Due in part to trade negotiations, regional harmonization and positive economic trends, the pharmaceutical market in Thailand is predicted to double by 2022.There are several versions of the Drug Act currently in effect, and the Thai government is working on a revised version with updated regulations. Under the current laws, pharmaceuticals are categorized as either traditional or modern medicines, with different applications and oversight. Modern medicines are subdivided into three categories, each of which has separate registration requirements. Licenses currently do not require renewal.

link……….http://drug.fda.moph.go.th/eng/

Thai FDA intends to accept dossier in eCTD format: The Drug Regulatory Authority of Thailand (Thai FDA) has initiated the acceptance of Pilot eCTD from October 2014.Read More
eCTD requirements
http://drug.fda.moph.go.th/eng/files/2_eSubmission%20FAQ1_0921.pdf
http://drug.fda.moph.go.th/eng/files/1_TH%20Module%201%20and%20Regional%20Specification_0921_Tch.pdf
http://drug.fda.moph.go.th/eng/files/TH%20Regional%20Specification%20and%20Validation%20Criteria.pdf
Step to be followed to submit eCTD application
Taken from
 Amar Tandon
Amar TandonA) Prepare Application to get a eSubmission Identifier for every application issued. A request to the THAI FDA online service should be submitted to obtain an eSubmission identifier which will require following details.
The eSubmission Identifier will be issued within 10 days of application. The Applicant must then make an appointment for submission within 30 days.
B) Prepare valid application along with validation reports as per country (Thailand) specific requirement with regional eSubmission Identifier provided.
The M1 requirements to be kept in consideration while compiling the Submission.
C) Dispatch Activity Delivery of the application at Thai FDA in CD/DVD (make an prior appointment with HA at drug_esubmissions@fda.moph.go.th
Thai FDA has proposed a set of media formats to be used while submission of eCTD
Future Aspect-Import: The eCTD will be validated and imported into the THAI FDA Review System
Feedback: Application feedback (if there are problems experienced during the upload) and review of application by Thai FDA
Ensure that you do not use. 1. Double-sided discs 2. Re-writable disc (protection, authenticity and Stability of information cannot
Ensure that you do not use:
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
///////
In 2014 the European Medicines Agency (EMA) issued the Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities. This publication triggered a discussion about the Permitted Daily Exposure (PDE) values in the Pharmaceutical and even in the API Industry, especially regarding crosscontamination and cleaning validation. Now a draft of a Q&A paper from the EMA provides some concretisation.

In 2014 the European Medicines Agency (EMA) issued the Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities. As mentioned in the publication itself, this document triggered a discussion about the Permitted Daily Exposure (PDE) values in the Pharmaceutical and even in the API Industry, especially regarding crosscontamination and cleaning validation. Now, the draft of a question & answer paper from the European Medicines Agency provides some concretisation of the guideline.
The document altogether comprises five pages with 14 questions and answers.
The questions – and even more the answers – are very interesting, as shown in question 1 already: Do companies have to establish Health Based Exposure Limits (HBELs) for all products?
The answer is: Yes, but there are references to question 2 and 4 (and their respective answers). Question 2 clarifies what products/active substances are considered as highly hazardous. There are, among others, 5 groups listed, which products should be classified as highly hazardous (e.g.compounds with a high pharmacological potency, daily dose < 1 mg/day (veterinary dose equivalent 0.02 mg/kg)). For highly hazardous substances the answer yes in question 1 is expected. Even more interesting is the link to question and answer 4: Can calculation of HBELs be based on clinical data only (e.g. 1/1000th of the minimum therapeutic dose)? And the answer is yes, but only at designated circumstances. This means the products should have a favourable therapeutic index (safety window) and the pharmacological activity would be the most sensitive/critical effect.
Some further clarification regarding LD 50 is provided in Question 5 and the respective Answer: The use of LD 50 to determine health based limits is not allowed.
There are also more questions and answers regarding Veterinary Medicinal Products, the inspection of the competence of the toxicology expert developing HBELs, Occupational Exposure Limits, cleaning limits, Investigational Medicinal Products and paedric medicinal products and about Cross Contamination. Details will follow.
The document is still a draft and the industry has the opportunity to comment it until the end of April 2017. Let´s see what the final version will bring.
Please also see the draft Questions and answers on implementation of risk based prevention of cross contamination in production and ‘Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities’on the EMA website.
At ECA´s Cleaning Validation Course, 9-10 February 2017 in Heidelberg, Germany the EMA Q&A draft will also be discussed.
some pics






///////////EMA, Q&A , Health Based Exposure Limits, 1/1000 dose , criterion, Cleaning Validation,

In the beginning of 2015 the FDA has published a draft guideline about GMP for Combination Products. Now the final version has been published. What are the differences between the draft and the final version of the FDA Guideline for Combination Products?
In the beginning of 2015 the FDA has published a draft guideline about GMP for Combination Products. Now the final version has been published. What are the differences between the draft and the final version? In the following you will find an overview:
The final guideline has expanded to now 59 pages (draft: 46 pages). And also the number of footnotes increased from 85 (draft) to 147 (final).
In the table of content there are one new subchapter (II B Quality and Current Good Manufacturing Practice) and one new chapter (VII Glossary). Subchapter III C was expanded to definitions and terminology. In the following the table of content is listed:
I. Introduction
II. Background
A. Definition of a combination product
B. Quality and Current Good Manufacturing Practices
C. Overview of the final rule
D. The role of the lead center and other agency components
III. General Considerations for CGMP Compliance
A. Demonstrating compliance
B. Investigational products
C. Definitions and terminology
D. What CGMP requirements apply to a product or facility?
E. Control of changes to a combination product
IV. What do I need to know about the CGMP requirements specified in 21 CFR 4.4(b)?
A. Provisions from the device QS regulation specified in 21 CFR 4.4(b)(1)
B. Provisions from the drug CGMPs specified in 21 CFR 4.4(b)(2)
C. Combination products that include biological products and HCT/Ps
V. Application of CGMP requirements to specific types of combination products
A. Prefilled syringe
B. Drug-coated mesh
C. Drug Eluting Stent (DES)
VI. Contact Us
VII. Glossary
VIII. References
In the introduction it is explicitly stated, that “The final rule did not establish any new requirements”. In a footnote the guideline gives an explanation why the term “legacy” combination product has not been used.
In the new subchapter II B (Quality and Current Good Manufacturing Practice) the guideline mentions, that “the core requirements embedded in these regulations provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. This includes establishing a strong quality management system, using appropriate quality raw materials, establishing robust manufacturing and control procedures based on sound design principles, and detecting and investigating product quality deviations. In addition, these regulations call for ongoing assessment of systems and the implementation of corrective actions where appropriate”.
The final document introduces in Section C the new term “CGMP operating system”. This means the operating system within an establishment that is designed and implemented to address and meet the current good manufacturing practice requirements applicable to the manufacture of a combination product. A clarification about constituent parts of cross-labeled combination products is also implemented. Further, there is a new passage about the choice of the GMP-approach (QS regulation vs drug CGMPs) also regarding a streamlined approach and for companies manufacturing different products. Completely new is the passage with the title “Documentation of CGMP Approach”. Here you can also find hints that manufacturerers with products that have been on the market since before GMP for Combination Products (21 CFR 4) came into operation, have to be compliant too. The guideline requires that the information about the “CGMP operating system” should be shared with FDA investigators in the beginning of an inspection.
In the “Demonstrating compliance” subchapter (III A) there is additional information about crossreferenced approaches (21 CFR 820 vs 21 CFR 211 and vice versa). For investigational products (III B) you can find more detailed information about exemptions from part 820 regarding 21 CFR 820.30 (Design).
In the Definition and terminology section (III D) there are amendments regarding container closure aspects and kits. Section III D (What CGMP requirements apply to a product or facility?) details the responsibility of the owner of a combination product and CAPA procedures in shared facilities.
In section III E. (Control of changes to a combination product) information for single entity and co-packed combination product manufacturers has been amended. The passages in IV A (Provisions from the device QS regulation specified in 21 CFR 4.4(b)(1) with regard to 21 CFR 820 about Management Responsibility, Design Controls, Purchasing Controls and CAPA have been extended – including examples – and “modernised”. Terms like quality oversight and QTTP are now mentioned there. Vice versa the passages with regard to 21 CFR 211, 211.84. 211.103, 211.132, 211.137, 211.165, 211.166, 211.167, and 211.170, (IV B Provisions from the drug CGMPs specified in 21 CFR 4.4(b)(2)) have also been extended – likewise with examples – and have been “modernised” as well (e.g. parametric release is mentioned).
In the example about prefilled syringes (V A) one can find an amended passsage about Design Controls and a new section about Design History File. In the example about drug-coated mesh (V B) there has also been included a new section about Design History File. In the drug eluting stent example (V. C) there are amendments in the section about 21 CFR 211.184, 21 CFR 211.103 and 21 CFR 211.170. Furthermore all examples comprise editorial changes.
Completely new is the chapter VII (Glossary). The number of references (Chapter VIII) increased to 31 (draft: 19).
Summary:
There are a lot of changes from the draft to the final document. One chapter (Glossary) and a subchapter ( Quality and Current Good Manufacturing Practices) are new, but there are also new passages and amendments in the final document. Helpful are the examples that have been integrated.
Please also see the Guidance for Industry and FDA Staff: Current Good Manufacturing Practice Requirements for Combination Products for more details.
///////////
