ICH
Source: ich.org
What is ICH?
- ICH is The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- It is unique project that brings together the regulatory authorities and pharmaceutical industry of Europe, Japan and the US to discuss scientific and technical aspects of drug registration.
- It was formed to achieve greater harmonisation in the interpretation and application of technical guidelines and requirements for pharmaceutical product registration in EU, Japan and USA
In Simple words ” ICH was formed to have a common scientific and technical requirements for registration of pharmaceuticals in EU, Japan and USA to make the job easy for Regulatory agencies and Pharma companies“.
Benefits of Harmonisation/Benefits due to the formation of ICH-
Harmonisation is beneficial to both regulatory authorities and the pharmaceutical industry, ultimately having beneficial impact for the protection of public health. The benefits of harmonization are listed below-
- Preventing duplication of clinical trials in humans and minimising the use of animal testing without compromising safety and effectiveness.
- Harmonisation would lead to a more economical use of human, animal and material resources, and the elimination of unnecessary delay in the global development and availability of new medicines while maintaining safeguards on quality, safety, and efficacy, and regulatory obligations to protect public health
-
Streamlining the regulatory assessment process for new drug applications; and reducing the development times and resources for drug development.
Origin Of ICH-
- Harmonisation of regulatory requirements was pioneered by the European Community (EC), in the 1980s, as the EC (now the European Union) moved towards the development of a single market for pharmaceuticals. The success achieved in Europe demonstrated that harmonisation was feasible.
- At the same time there were bilateral discussions between Europe, Japan and the US on possibilities for harmonisation.
- It was, however, at the WHO Conference of Drug Regulatory Authorities (ICDRA), in Paris, in 1989, that specific plans for action began to materialize.
- Soon afterwards, the authorities approached IFPMA to discuss a joint regulatory-industry initiative on international harmonisation, and ICH was conceived.
- The birth of ICH took place at a meeting in April 1990, hosted by EFPIA in Brussels. Representatives of the regulatory agencies and industry associations of Europe, Japan and the US met, primarily, to plan an International Conference but the meeting also discussed the wider implications and terms of reference of ICH.
-
At the first ICH Steering Committee (SC) meeting of ICH the Terms of Reference were agreed and it was decided that the Topics selected for harmonisation would be divided into Safety, Quality and Efficacy to reflect the three criteria which are the basis for approving and authorising new medicinal products.
ICH Secretariat-
ICH does not have “offices” as such, but ICH secretariat is based in Geneva, Switzerland. The biannual meetings and conferences of the ICH Steering Committee rotate between the EU, Japan, and the USA.
ICH does not have “offices” as such, but ICH secretariat is based in Geneva, Switzerland. The biannual meetings and conferences of the ICH Steering Committee rotate between the EU, Japan, and the USA.
Members of ICH-
MHLW – Ministry of Health, Labour and Welfare
JPMA– Japan Pharmaceutical Manufacturers Association
EU– European Union
EFPIA– European Federation of Pharmaceutical Industries and Associations
FDA– Food and Drug Administration
PhRMA -Pharmaceutical Research and Manufacturers of America
MHLW and JPMA are from Japan
EU and EFPIA are from Europe
FDA and PhRMA are from USA
EU and EFPIA are from Europe
FDA and PhRMA are from USA
Non Voting Members of ICH-
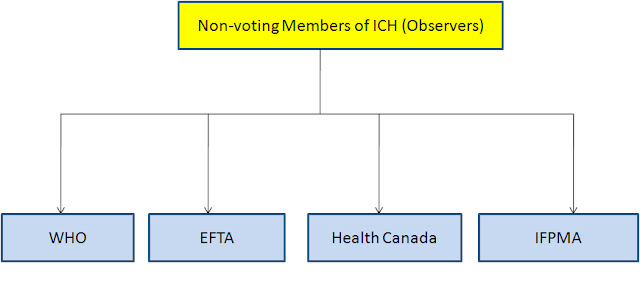
WHO-World Health Organization
EFTA-European Free Trade Association
IFPMA– International Federation of Pharmaceutical Manufacturers & Associations
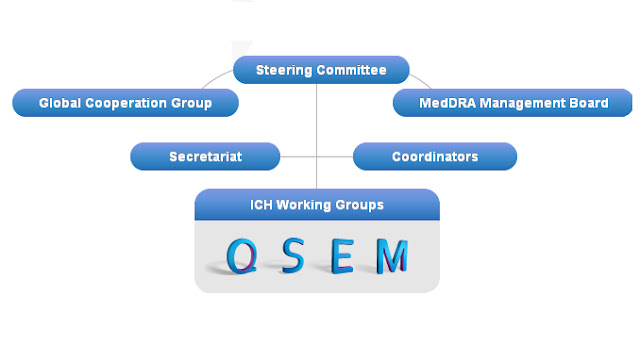
Organisation of ICH
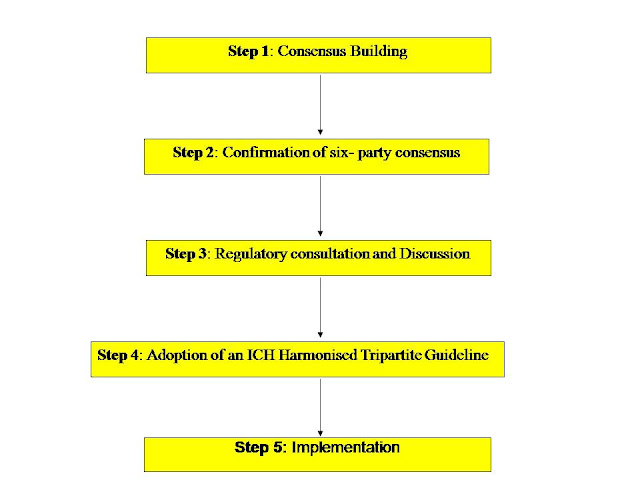
Process of Harmonisation:
ICH harmonisation activities fall into 4 categories: Formal ICH Procedure, Q&A Procedure, Revision Procedure and Maintenance Procedure, depending on the activity to be undertaken (see below).

Formal ICH Procedure for new topic of harmonisation :
The Formal ICH Procedure is a step-wise procedure consisting of 5 steps .This procedure is followed for the harmonisation of all new ICH topics
ICH guidelines have been adopted as law in several countries, but are only used as guidance for the U.S. Food and Drug Administration
1. Explanation of Abbreviations quoted ABOVE-
(i)MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology used by regulatory authorities and the regulated biopharmaceutical industry throughout the entire regulatory process, from pre-marketing to post-marketing activities, and for data entry, retrieval, evaluation, and presentation.
(ii)
Q-Quality
S-Safety
E-Efficacy
M-Multidisciplinary
(iii)Q3C is – Impurities :Guideline for Residual Solvents
(iv) M2 is -Multi-disciplinary Group 2
References :
1.http://www.ich.org/
2.http://www.wikipedia.org/
30,617 total views, 1 views today













