Contract API Manufacturing, Custom synthesis
A new report forecasts the world market for pharma contract manufacturing will generate $79.24bn in 2019. Contract active pharmaceutical ingredient (API) and finished dosage formulation (FDF) manufacturing will experience strong revenue growth in the first half of the 2013-2025 forecasting period. Although generic APIs will remain as the main contributor in that production services industry, demand for highly potent APIs (HPAPIs) will increase in this forecast period, with a revenue growth CAGR of 7.7%. Those predictions and others appear in Pharmaceutical Contract Manufacturing: World Industry and Market Outlook 2015-2025, published in February 2015.
That new investigation finds API manufacturing services accounted for the largest proportion of contract manufacturing organisation (CMO) revenue in 2014. This will continue to be the case throughout the period 2015-2025. Demand will be strong for highly potent APIs in developed national markets. That trend encourages CMOs to invest in upgrading their facilities to offer manufacturing capacity in HPAPIs. Fastest growth in the worldwide contract manufacturing market will come from the contract FDF manufacturing sector. That submarket’s revenues will grow with a CAGR of 6.8% between 2013 and 2025, the study finds.
Sandra Wenas, a pharmaceutical industry analyst, said: “The pharmaceutical contract manufacturing industry will continue to achieve consolidation via mergers and acquisitions (M&A). Consolidation is expected to peak in the next five years, as more CMOs strive to provide a one-stop-shop, offering services from APIs to finished doses. Excess capacity is also the driver of the recent M&A activities.
“Affordability is no longer the main parameter in selecting CMOs for partnership, as sponsor companies will increasingly emphasize quality, compliance with regulatory demands and service requirements. Technological advances will also stimulate that market from 2015 to 2025. Also CMOs invest in single-use technologies for biopharmaceutical manufacturing and continuous manufacturing processes. CMOs will also invest heavily to expand their capacities in HPAPIs.”
Along with prediction of the overall world market for outsourced drug production, the new report gives revenue forecasting of 11 world-level submarkets to 2025:
- Active pharmaceutical ingredients (APIs), with sub forecasts for generic APIs, high potency active pharma ingredients (HPAPIs) and other products
- Finished dosage formulations (FDFs), with sub forecasts for solid dose forms, injectable dosages, and other agents
- Other applications of outsourced production
- Clinical manufacturing
- Commercial manufacturing.
In 2015 commercial manufacturing projects account for most CMO revenue. However, improved funding for early-stage projects in the US and EU will drive demand for clinical trial material manufacturing towards the middle of the forecast period. The growing R&D pipeline of novel antibody-drug conjugates (ADCs) will also create more opportunities for CMOs offering specialised manufacturing technologies, further stimulating the market.
the reports new study also discusses regulatory developments and demand for pharma contract manufacturing services in leading and emerging national markets. That work analyses the US, Japan, Germany, France, Italy, Spain, the UK, China, India, Brazil, Russia and South Korea, with revenue forecasting to 2025. Developed markets account for the greatest demand for outsourced drug manufacturing. The US and EU accounted for 63.9% of CMO revenues in 2013. And concerns with regulatory compliance led some Western companies to seek outsourcing partners closer to home. Emerging national markets, however, will still generate much business from pharma companies wanting custom medicine production.
That investigation provides quantitative and qualitative analyses of the pharma contract manufacturing market for the period 2015 to 2025. It also shows interviews with companies. Pharmaceutical Contract Manufacturing: World Industry and Market Outlook 2015-2025 adds to the reports portfolio of market analyses on pharmaceutical outsourcing and other healthcare industries.

With technology racing onward, drugs are becoming more complex than ever before – along with theiractive ingredients and the processes that produce them.
 Different Challenges, Different Scales – The Balancing Act
Different Challenges, Different Scales – The Balancing Act
To some extent, the challenges of commercial API manufacturing vary, based on scale. A large-scale CMO might not necessarily be suited to working in an early stage of development. Applying later-stage standards early on, for example, could waste time and money – ultimately derailing the project timeline and budget. At the same time, consideration must always be given to later-stage manufacturing processes & requirements during earlier phases of the project to maximize efficiency and minimize cost & time-to-market. It requires striking a balance – maximizing early-stage success while minimizing challenges in the future.
Scale-Up Gradually to Identify & Solve New Challenges at Each Stage
Many aspects of early-stage production, such as handling and storing raw materials, only become challenging when production is scaled up. Issues with heat generation and dissipation also tend to come to the forefront when production is dramatically increased. In addition, larger volumes often mean longer reaction times. This can be problematic for certain processes.
To detect and resolve these potential issues, production is scaled gradually, going from micro or milligram scale to gram scale, then pilot scale to full production.
Lifecycle Management Key to Successful Process Scale-Up
When preparing to scale up drug production, it’s important to partner with a CMO well-versed in lifecycle management. A contract firm that has the extensive expertise needed to work comfortably at all scales can improve many aspects of the scale-up process in measurable ways, making it smoother, faster, and easier overall.
The issues that arise when shifting from one scale to another can be unpredictable, and because of this,planning and forecasting should be done as early in the R&D process as possible. The more experienced thetransfer team, the more streamlined and simplified the technology transfer can be.
Several techniques are currently used to make production more efficient. Scouting alternate synthetic routesto a molecule has become standard, and helps control costs by accelerating production. This can also reduce time spent on regulatory compliance, as there are less steps to document, and less analysis to be made throughout the process.

Consider Your Infrastructure: Analyze Processes, Steps, Chemicals & Reagents
We often manage projects with compounds from the earliest stages in which compounds are produced in small trial batches. With subsequent scale-up steps on the horizon, however, we always advise our earlier stage clients to consider the discharge, infrastructure and cost of the processes used. We systematically analyze how each process, step, chemical, or reagent can potentially affect the product when it begins manufacture on a larger scale. One solution we and other API manufacturers are increasingly turning to is green chemistry.
In green chemistry:
- Processes are designed to maximize the amount of raw materials in usable products
- Operations are designed to use less energy and to maximize energy efficiency overall
- Environmentally safe or benign products are used whenever possible
- Waste is avoided as much as possible
By following these principles, the processes used for larger scale API manufacturing can become more efficient.
- Reactions use less steps, and fewer resources are consumed to yield more product.
- Operations are simpler, and conducted at ambient temperature and pressure.
- Workplaces became safer overall, as they house less toxic solvents and less hazardous waste products.
- Processes require less heat, less pressure, and less protective equipment.
The reduction in hazardous materials and wastes also helps decrease the time spent tracking, managing, and reporting on safety.
While always beneficial for Environmental Health and Safety (EHS) reasons, green chemistry can significantly reduce waste and cost. In addition, it can also be the key element that enables a drug to be manufactured commercially.



The Right Route Can Deliver Fewer Steps, More Consistent Batches, Cleaner Processes…and Cost Savings
With drug development and manufacturing processes becoming more complex, alternate synthetic route design is often a critical step where existing methods are impractical for large scale production.
Custom synthesis and route scouting is a big part of . Synthesis scouting has grown in importance as the benefits of taking action (and the downsides to convoluted, difficult and lengthy synthesis processes) become more apparent.
Route Steps – Combine, Reorder, Reduce, Modify and Simplify.
Synthetic route scouting brings together a number of disciplines, including medicinal-, synthetic- and preparative-chemistry. All aspects of the drug’s chemistry must be thoroughly explored. Then we must understand what happens at each step in the route – before any step is combined and an alternate, shorter route can be designed.
In other cases, alternate route design considers the order of events. In a previous post, we referenced the importance of when certain chemicals or reagents are added to the process. As I mentioned in that post, theorder of events can spell the difference between a successful drug and a failed candidate.
Rethink the Route.
There are a number of specific reasons to develop alternate synthesis routes, many of which can directly impact safety, efficacy, and manufacturing efficiency & sustainability. Here are two key aspects to “Rethinking the Route,” both of which can contribute to significant advantages – whether cost-, safety or infrastructure based.
Solvent Selection
The chemicals and reagents used to synthesize a molecule invariably come under the microscope during alternate route scouting. Solvent selection can have a wide-ranging impact on manufacturing cost andhazardous waste manufacturing infrastructure, and can add additional processing steps in order to clear toxic compounds or byproducts.
In many cases, front loading any potentially toxic or hazardous chemicals as early in the process as possible is a good option to allow sufficient clearance time. Early introduction is a fundamental strategy in cases where the number of processing steps is significantly reduced, since the solvents may still require multiple steps to allow for sufficient contaminant clearance.
At Neuland we try – as much as possible – to use universal solvents (including water) that have well-characterized safety profiles, and that tend to be:
- less toxic
- easier to manage or treat
- widely available
- less expensive
By selecting the proper solvent and introducing it at the optimal processing step, a route change can deliver big benefits, including a shorter process, cost savings and cleaner, more consistent batches.
Cleaner processing with fewer or less-toxic solvents also deliver “green” savings on the back end, with hazardous waste management, treatment & disposal, safety, and other EHS-related subjects.
Take the Shorter Route
Route shortening is almost always a key objective of alternate route scouting. Fewer processing steps can have an enormous impact in terms of time, regulatory challenges, cost and infrastructure (including waste handling – discussed above).
A shorter route is often an efficient route. Fewer steps mean fewer critical control points. It means potentially improved consistency, with fewer opportunities for out-of-spec product. It means less processing infrastructure – equipment, utilities, manpower, time, and more. It can bring unwieldy processes in-line with the ever-increasing price pressures on drugs.
Route scouting is critically important to the design of effective, economical and efficient manufacturing processes that help improve drug company or pipeline sustainability.
The pharmaceutical contract manufacturing market is expected to hit revenues of $80.5 billion in 2019, according to a report by business intelligence provider Visiongain (1). A notable trend is that pharmaceutical companies will continue to outsource more drug production operations. For several years, API manufacturing has formed the largest share of the pharmaceutical contract manufacturing market, driven by the increased use of generic drugs worldwide, the rise of biologics and biosimilars, and the growth of emerging markets such as India and China (1).
To remain competitive in an increasingly demanding pharmaceutical market, contract service providers are constantly striving to increase the capacity and efficiency of their manufacturing activities. Pharmaceutical Technology spoke to industry experts about process optimization in API manufacturing. Participants in this roundtable discussion include Joshua P. Van Kley, corporate sales and sales operations manager, Cambrex; David Goeddel, PhD, group leader, Process Development, MilliporeSigma;
Andreas Stolle, vice-president, Process Development Services API, and Peter Poechlauer, innovation manager, who are both based in Linz, Austria, which is currently Patheon’s largest API manufacturing site.
Developing an API manufacturing process
When developing an API manufacturing process, there are a number of important considerations from a practical and logistical standpoint. Firstly, can the chemistry be performed at the manufacturing site in terms of handling the necessary solvents and reagents, and does the plant have the capabilities to accommodate the temperature ranges of the process? It is also important to evaluate the specific hazards and safety implications of undertaking the process.
Availability of key raw materials must be evaluated to ensure that they are readily available from existing suppliers, or whether new suppliers can be established, to avoid a situation where you are limited by supply of a key raw material or unable to import it.
It is also important to look at the process from an environmental point of view, to ensure that all waste can be handled and disposed of properly, and also to ensure that the process is scalable from laboratory through to the commercially projected scale. Hazards, by-products, and waste products that are not as consequential at smaller scale can become major issues at large scale, thus, it is important to factor these considerations in from the beginning.
There are also many other considerations that come into play, such as clinical phase, cycle time, the control of the product’s particle size, polymorphism, and handling issues such as the filterability of steps within the process. All these factors can have an impact on the quality of the product as well as cost of goods, therefore, it is important to bear them in mind when providing a quality product as well as meeting the customer pricing demands.
Several factors should be taken into account when developing the manufacturing process for an API. Careful focus should be placed on ensuring that the overall purity, purity profile, and individual impurity levels are at acceptable levels to ensure the safety of the patient. Guidance has been provided in this area by the International Council for Harmonization (ICH) on threshold limits for impurity identification and qualification in API drug substances–ICH Q3A. In addition to organic impurities, process chemists should also pay close attention to residual solvent levels and elemental impurities when developing a process for API manufacturing. This factor is particularly important when metal catalysts are used in the API synthesis, and remediation techniques (scavengers, charcoal, or crystallization) are often required to reduce these impurities to the acceptable levels outlined in ICH Q3D.
In addition to impurities, another aspect that needs to be taken into consideration when developing an API process is the potential reactive hazards. Performing a thorough safety evaluation and modifying the chemistry as appropriate will enable the API to be made safely, which will help prevent operator injuries, plant or equipment damage, and potential supply-chain interruptions. The raw material supply chain is another important factor. Not only does the vendor need to be qualified, but they must also be able to ensure the long-term timely delivery of needed raw material quantities in the required quality.
Finally, throughput is another important factor that should be taken into account when developing a process. Improvement in yield lowers API cost and reduces waste, which will consequently benefit the patient, company, and the environment.
API purity, impurity levels, raw material supply chain, yield, and process safety are all important factors that should be taken into account when developing a process for API manufacturing. Focusing on those key areas will help secure both patient and employee safety while completing efficient chemical syntheses that reduce cost and minimize the impact on the environment during API production.
The development of a
pharmaceutical manufacturing process has to meet different requirements depending on the development phase of the product:
- In early clinical development (CT I), the primary goal is to deliver the required amounts of material quickly and in reproducible quality.
- Later on (in CT II), when the route is frozen, the production process must be reliable, well understood, and again deliver the product in the required quality. The appearance of potentially genotoxic byproducts in the final product must be excluded in a safe and scientifically sound way.
- Finally, the process for the final clinical trials (CT III) and launch of the product must be scalable to deliver the required product volumes with predictable quality, and it has to be environmentally benign and economical on resources.
- After product launch, the process must have room for continuous improvement without major changes.
In addition to the process figures, the following factors must be taken into account:
- Availability and safe supply of starting materials in constant quality
- Investments in equipment to operate the envisaged process at the desired scale
- Start-up time and time demand for capacity changes
- Easy process transfer between different manufacturing sites to meet local demand and support supply-chain optimization.
The current regulatory environment supports advancing regulatory science and innovation, which may include abandoning some traditional manufacturing
practices in favor of cleaner, more flexible, and more efficient continuous manufacturing. Regulatory authorities in the three ICH regions and beyond are encouraging the industry to adopt new technology as supported by ICH Q8(R2), Q9, Q10, and Q11; and the introduction of quality-by-design (QbD) concepts, emphasizing science and risk-based approaches to assure product quality. The regulatory expectations for assurance of reliable and predictive processing, which is technically sound, risk-based, and relevant to product quality in a commercial setting, are the same for batch and continuous processing.
Optimizing process chemistry
How do you optimize process chemistry in API manufacturing? What are the key considerations?
Our key considerations in optimizing process chemistry are driven by optimizing the service to our clients. This approach comprises considerations such as:
Chemicals and reagents:
- Are the involved chemicals and reagents reliably available?
- Is their price acceptable in view of the target product price?
- Are the solvents recyclable?
- Does the generated waste pose a problem?
- Is it biodegradable?
Process conditions:
- Can we develop a sufficiently detailed process
- understanding?
- Do the chosen conditions allow quick and safe scale-up?
- Is the process easily transferable between sites or does it require specialized equipment?
Batch-wise versus continuous processing concept:
- Will the process yield/throughput profit from continuous manufacturing techniques?
- Will process control profit from continuous manufacturing?
- What are the options for rework?
Process control:
- Can we develop a sufficiently quick and easy process control system?
- Are the chosen analytical methods reliable and easily transferable?
- Does the process control system support continuous process verification?
Our optimization strategy comprises both classical determination of proven acceptable ranges (PAR) values and, in tight collaboration with clients, strategies of multivariate analysis and other elements of process analytical technologies. In addition to technical aspects of optimization, there are aspects related to client requirements, such as use of innovative but proven technologies to provide maximum value.
Initially, the process is carried out in its current state using the conditions provided by our clients. This approach allows us to observe the chemistry and get a feel for how it performs. From there, the next stages of development investigate ways to reduce solvent volumes, increase yields, reduce cycle times, lower raw material costs, and lower waste costs. These steps are crucial to improving product quality and the economics of the process, which allows us to pass efficiencies and qualityon to our clients. Most of this work is undertaken in the chemical development laboratory prior to going into production. Once in production, the chemist and engineer assigned to the program will further work on optimization of the process based on observations made during production. In addition, our continuous improvement/six sigma group will also contribute to the optimization process once the program is in validation or commercial launch. The group will help in managing the lifecycle of the program along with looking at ways to continually improve the efficiency of production by data mining.
Typically, we will see programs that have chromatography steps within the process, high volume issues, filtration issues, and/or long cycle times. Our development efforts are centered on removal of any chromatography processes if present for scaling purposes, volume reductions, faster filtrations, and cycle time reduction, either for efficiency or the possibility of telescoping steps to reduce unnecessary isolation steps if the process lends itself.
We strive to perform phase-appropriate process optimization for API manufacturing. Process optimization means very different things for Phase I clinical programs compared with programs that are entering validation. For an API that will be entering Phase I, the key objective is usually to rapidly develop a process that can safely yield the required API with the necessary quality attributes. This way, clinical evaluation of the API can begin quickly, which is important for both drug developers and patients who seek successful treatment. As the program advances toward validation and commercial launch, greater emphasis is placed on improving yield and gaining greater process understanding to support process validation and eventual launch.
No two programs are the same, but there are some consistent factors that generally apply to most programs. We consider several factors when deciding whether the incoming synthetic route can be used or if a new synthesis should be developed. Raw material supply chain, process safety, projected future API manufacturing costs, likely commercial scale, and timing all play an important role in the decision-making process. After a route is selected, proof-of-concept studies are performed to determine whether or not the proposed route can generate the API. We then optimize the process to reliably and safely generate API in adequate quality. This objective is achieved by building process understanding through many techniques, including impurity origin and control, identifying critical parameters, and setting appropriate limits on operating ranges. We develop this chemistry with an eye on the intended commercial manufacturing scale, because APIs that will be manufactured on a smaller scale will have more processing options available than those that will be made on a larger scale. During the final phase of optimization, experimentation is performed to determine if the API can consistently be manufactured with the required quality attributes. Statistical design of experiments is a particularly useful technique for these studies, because interdependent variables can readily be identified. The successful completion of these phase-appropriate process optimization efforts enables us to deliver high-quality clinical batches and commercial supply in a timely manner, which is important for our customers and their patients.
Manufacturers take steps that span from early research and development through commercial manufacture to ensure that APIs of consistent quality are produced. The general pathway for this process is outlined by FDA and involves three phases: process design, process qualification, and continued process verification.
During the process design phase in development, great effort is made to understand what parameters are critical. Building upon that knowledge, the process is optimized as necessary to enable the desired quality attributes to be consistently achieved. Manufacturers then perform a failure modes effects analysis (FMEA) on the process to identify processing risks that could impact quality attributes. From that exercise, additional experiments can be designed to address risks identified in the FMEA to ensure that the critical quality attributes are reliably met. During this stage of development, manufacturers perform stress testing, stability studies, design of experiments, and range-finding studies to help ensure that the intended quality is
consistently produced in subsequent manufacturing.
The process qualification phase involves an assessment of whether or not the process is reproducible. There are two major components to process qualification. The first part involves the qualification of the plant and equipment to ensure everything works as intended. The second part involves the qualification of the process itself through an activity known as process performance qualification (PPQ). PPQ involves drafting a protocol, execution of the protocol for the specified number of batches under current good manufacturing practices (cGMPs), and issuance of a report. Following successful completion of the process qualification, the process can be used for commercial supply of the API.
The process to ensure product quality does not end with product launch. Manufacturers use systems that enable them to track process data and identify any sort of trend that may require intervention. Furthermore, an adequate facility and equipment maintenance program ensures that the plant and equipment are functioning at the desired level. By performing all of the aforementioned activities, manufacturers can ensure that APIs of the intended quality are consistently produced.
The key to consistent quality product is a sound process understanding combined with effective process control. Process understanding suffers if the features of the processing equipment mix with features of the actual chemical reaction, blurring them and interfering with precise process control. Consistent production of APIs of intended quality starts with a kinetic and thermodynamic analysis of the synthesis reaction. The rate, energy balance, and kinetics of by-product formation and factors such as equilibria of phase distribution determine the requirements of the process. They in turn determine the features of the processing equipment and ultimately the equipment selection. They also determine the control strategy to effectively safeguard consistent product quality. A sound process understanding allows the
conscious choice of proven acceptable ranges for reaction parameters and intermediate product quality. It avoids overly narrow parameter ranges or unnecessary tight intermediate product specifications and thus allows for continuous improvement without putting API quality at risk. In many cases, continuous processing simplifies the precise control of process conditions even for processes that are very exothermic or require quick mixing to establish the correct stoichiometry and avoid byproduct formation.
Continuous processing equipment can be tailored to meet the respective requirements of a chemical reaction or work-up section with moderate effort. Its combination with state-of-the art methods of continuous analytics allows precise and reliable control of product quality.
There appears to be a paradigm change: instead of slowing down the chemistry to a degree to allow large-scale batch processing equipment to cope with heat evolution etc., the developer determines ideal conditions for the respective chemical transformation and defines (or if necessary constructs) suitable processing equipment and control instruments. This approach leads to more modern ways of processing and, as the PAT guideline (2) stipulates, ‘development and implementation of innovative pharmaceutical development, manufacturing, and quality assurance.’
An important step is performing critical process parameter studies. A critical process parameter study is initiated to identify those critical parameters in the process that affect the final product quality and reproducibility. Typical critical parameters to be investigated at each step requiring validation may include: temperature, charge ratios, pressure, vacuum, time (duration), flow rate, cooling rate, and agitation speed, among others.
Critical process parameters are usually identified and studied after initial laboratory work, or after initial manufacturing campaigns, when the chemists can observe the behavior of the chemistry at scale. Our customers typically dictate when they want this work to be performed to tighten up the operating parameters.
From a quality perspective, Cambrex follows ICH Q7 guidelines. In addition, we have a strong analytical method validation program in place for all analytical methods, including cleanout methods for each isolated intermediate as well as finished goods. The validated cleanout methods not only ensure quality for the current product being manufactured, but also ensure the quality and integrity of the plant for the next product to be produced, as we operate a multipurpose facility with non-dedicated production streams.
References
1. Visiongain, “Pharma Contract Manufacturing Services Market Will Reach $80.5 Billion in 2019,” accessed July 19, 2016.
2. FDA, Guidance for Industry, PAT–A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (Rockville, MD, September 2004).
Article Details
Pharmaceutical Technology
Vol. 40
APIs, Excipients, and Manufacturing Supplement
September 2016
Pages: s6–s10
Citation
When referring to this article, please cite it as A. Siew, ” Optimizing API Manufacturing,” APIs, Excipients, and Manufacturing supplement to Pharmaceutical Technology 40, 2016.
A PRESENTATION
 The presentation will load below
The presentation will load below
Custom Manufacturing Services:
Exactly What You Need, Delivered Where and When You Need It
Case study
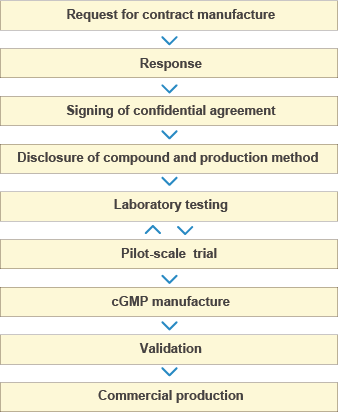
Few decisions in pharmaceutical operations are as critical as selecting the right cGMP Contract Manufacturer for an API. APIs manufactured to cGMP standards for commercial sale must meet requirements for identity, strength, quality and purity.
CordenPharma is linking together a legacy of expertise to provide reliable, effective cGMp contract manufacturing of APIs to support your small and large-scale projects through:
- Big Pharma / Biotech calibre facilities with stringent quality systems and standards
- Proven track record of success in API small and large-scale manufacturing, including thelargest peptide API production capacity worldwide
- Network of global cGMP manufacturing facilities with combined capabilities and successful track record of inspections by all relevant Health Authorities
- Dedicated project managers supported by a project team to ensure consistent coordination, communication and alignment of progress throughout all project phases
We conduct commercial cGMP manufacturing of APIs and advanced intermediates for bothclinical trial supplies and commercial products. Moreover, we are uniquely suited to provide the full spectrum of cGMP synthesis and chemical manufacturing services, from production of early stage clinical trial materials to product launch and commercialisation.
Our technical manufacturing capabilities span a wide range:
- Reactors pools with high flexibility in size (100-18000L ), media compatibility (Hastelloy; glass-lined, steel) and temperature (-100°C to +200 °C)
- Multiple product isolation technologies (centrifuge, filters) with wide material compatibility (Hastelloy; stainless steel)
- Multiple drying options (agitated and static dryers)
- Multiple powder handling and finishing capabilities including sieving, blending and milling
- Handling of highly potent compounds down to exposure limits of 1ng/m3
CordenPharma’s comprehensive repertoire of chemical reaction types at industrial scale includes almost all chemical transformations in use today such as:
- Large-scale hydrogenations, including enantioselective hydrogenations up to 60 bar
- Industrial-scale enzymatic reductions
- Industrial-scale chemical peptide synthesis, both solid and solution phase
CordenPharma’s full range of API Contract Manufacturing and Development Materials Includes:
- Route Scouting & Process Development
- Small Molecules
- Peptides
- Conjugates
- Lipids
- Carbohydrates
- cGMP Large-scale synthetic Peptide API Production
- Solid phase and solution phase synthesis
- Peptidomimetics, lipopeptides
- cGMP Small-molecule API Manufacturing
- Pharmaceuticals
- Fine chemicals
- Highly Potent APIs (OEL 4)
- Amino Acid Derivatives and Building Blocks for peptide synthesis
- Natural and non-natural amino acids
- Pseudoproline dipeptide building blocks
- Derivitized Phospholipids and other Lipids
- Hetero and homogeneous, conjugates
- Helper lipids, sphingolipids, cationic lipids
- Carbohydrates
- Generic API Manufacturing
- Sterile APIs
- Standard Catalogue Products which are readily available:
- Manufacturing and Development Support for Contracted Customers
- Analytical method development and validation
- Regulatory support activities including Pharmaceutical Writing
37,142 total views, 1 views today












