CEP
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Organic or inorganic substances (active or excipients), manufactured or extracted.
- Sterile Active Substances
- Substances produced by fermentation as indirect gene products, which are metabolites of microorganisms, irrespective of whether or not the microorganisms have been modified by traditional procedures or r-DNA technology
- Products with risk of transmitting agents of animal spongiform encephalopathies (TSE)
- Herbal Drugs and Herbal Drug Preparations
- Direct gene products (proteins)
- Products obtained from human tissues
- Vaccines
- Blood products and preparations.
Guidelines to be referred for preparing Dossier to obtain a CEP
Content of the Dossier for Chemical Purity and Microbiological Quality (PA/PH/CEP 04 1 4R)
1. Each of the above guideline corresponds to individual type of substances. For example, the first Guideline is to be referred for preparing dossier to obtain a CEP for an API.
Link for Downloading Application form and template of QOS.
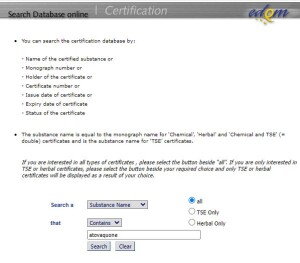
Link to Online Databases of Approved CEPs
Registration fees for various New CEP Applications Deficiencies
Link to top 10 deficiencies in New Applications for grant of CEPs Recognition of CEPs- CEPs are recognised by all the member countries of the European Union and also other Countries like Canada, Australia, New Zealand, Tunisia and Morocco.
Advantages of CEPs-
- It is valid for 5 Years.
- An API manufacturer need not file an ASMF/EDMF in all the member countries involved in DCP/MRP Procedures if the manufacturer has obtained CEP for that specific API. Sending a Copy of CEP will do.
- Centralised evaluation of Dossier by only one agency i.e EDQM.
References-
https://www.edqm.eu/en/certificate-suitability-new-applications
Certificate of Suitability: New Applications
Submit A New Application
To obtain a Certificate of Suitability to the monographs of the European Pharmacopoeia (CEP), applicants must send in electronic format the following documentation to the Certification of Substances Department (DCEP) of the EDQM:
- a completed application form which includes your invoicing details
- a dossier in CTD format written in one of the two official languages of the Council of Europe (preferably in English),
- a single copy of the Quality Overall Summary (QOS).
Upon receipt, the application is validated and listed for assessment. After assessment, the EDQM may send queries to the applicant. When they are resolved, the EDQM sends the applicant a CEP, which is valid for 5 years from the date of first issue and valid indefinitely following the 5-year renewal.
To ensure the official timelines are met, the EDQM implements a strict procedure for assessment of CEP applications.
The evaluation of new applications is handled with three rounds of assessment. A policy document describes this policy and provides clarification on the potential outcomes of assessment and subsequent activities. Applications lacking sufficient information after evaluation of the applicant’s response to a maximum of 2 EDQM deficiency letters are definitively closed.
Sister files
The sister files procedure is intended to facilitate the submission of similar dossiers within the Certification Procedure, and to allow applicants benefit from a fast-track procedure and harmonised assessments.
A company which has been granted a certificate of suitability (CEP) may wish to apply for another CEP for the same substance, either when it is not possible to apply for a revision of the initial CEP or when the company wishes to have separate CEPs for different conditions of preparation or qualities (for example, to cover an alternative manufacturing process, manufacturing site or an alternative grade).
This new application can be submitted as a “sister file”, provided that the conditions listed in the guideline are fulfilled.
A specific application form should be used for the submission of a sister file.
These applications are managed as described in the document Management of applications for new Certificates of Suitability, Requests for Revision or Renewal of Certificates of Suitability and applications using the ‘sister files’ procedure (PA/PH/CEP (13) 110, 3 R, December 2021).
Content of the Dossier
Detailed information on what an application should contain is described in the documents below. Refer to the document relevant to your application.
- Content of the dossier for chemical purity and microbiological quality, PA/PH/CEP (04) 1, 6R
- Content of the Dossier for a Substance for TSE Risk Assessment (PA/PH/CEP (06) 2 1R)
- Content of the Dossier for Herbal Drugs and Herbal Drug Preparation Quality Evaluation PA/PH/CEP (02) 6 1R
- Certificates of Suitability for Sterile Active Substances (PA/PH/Exp. CEP/T (06) 13, 1R)
You should also read the:
Submission Format
From January 2018 all submissions should be in eCTD electronic format except submissions for substances for veterinary use (vNees is accepted) or for TSE risk (DF is accepted).
From 1 January 2017 submissions should be sent via the “Common European Submission Platform (CESP)”. Users should first register with the CESP before sending submissions to the EDQM.
Instructions for submitting electronic documents using the CESP platform are available here: User guide (PA/PH/CEP (13) 67, 2R)
Please read the “Guidance for electronic submissions for Certificates of Suitability (CEP) applications”
Important information
CEP holders are obliged to inform all their customers for each CEP revision, suspension, withdrawal or negative outcome of an EDQM inspection.
Find a guideline
Please go to Certification Policy documents & Guidelines for a list of all of our guidelines.
Looking for a CEP?
Search for a list of granted CEPs, their type, the name of the substance, the full CEP number, the issue date and validity status, using the Certification Database (updated daily).

More information about Fees
Samples
Applicants commit to provide samples of substance and/or impurities when requested by the EDQM.
47,071 total views, 2 views today