http://www.leadformix.com/ef1/preview_campaign.php?lf1=900485181e199412625317a3906967
Click here to contact Zack Systems SpA about this product.
Bosentan is a dual endothelin receptor antagonist important in the treatment of pulmonary artery hypertension (PAH).
Bosentan belongs to a class of drugs known as endothelin receptor antagonists (ERAs). Patients with PAH have elevated levels of endothelin, a potent blood vessel constrictor, in their plasma and lung tissue. Bosentan blocks the binding of endothelin to its receptors, thereby negating endothelin’s deleterious effects.
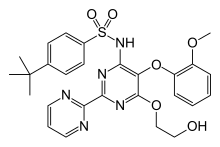
bosentan
Bosentan is a dual endothelin receptor antagonist used in the treatment of pulmonary artery hypertension (PAH). It is licensed in the United States, the European Union and other countries by Actelion Pharmaceuticals for the management of PAH under the trade name Tracleer.
Bosentan is indicated mainly for the treatment of pulmonary hypertension. In 2007, bosentan was approved in the European Union also for reducing the number of new digital ulcers in patients with systemic sclerosis and ongoing digital ulcer disease.Bosentan is a competitive antagonist of endothelin-1 at the endothelin-A (ET-A) and endothelin-B (ET-B) receptors. Under normal conditions, endothelin-1 binding of ET-A or ET-B receptors causes pulmonary vasoconstriction. By blocking this interaction, bosentan decreases pulmonary vascular resistance. Bosentan has a slightly higher affinity for ET-A than ET-B.
In the United States, bosentan is indicated for the treatment of pulmonary arterial hypertension (WHO Group I) in patients with WHO Class II-IV symptoms, to improve exercise capacity and decrease the rate of clinical worsening.[1]
Warnings
Due to potential hepatotoxicity, the FDA requires monthly monitoring of liver function tests while taking Bosentan.
Bosentan use requires hematocrit monitoring due to potential onset of anemia. [2]
Hormone-based contraception is not possible in women taking Bosentan, due to a pharmacokinetic interaction. [3]
Bosentan is absolutely contraindicated in pregnancy because of its teratogenicity. Category X.
- http://www.tracleer.com/pdf/09%20276%2001%2000%200809_Tra%20PI_4%20Pg_081409pdf.pdf
- http://www.ionchannels.org/showabstract.php?pmid=15875338
- http://www.ionchannels.org/showabstract.php?pmid=15875338





















 LinkedIn
LinkedIn Facebook
Facebook Twitter
Twitter GooglePlus
GooglePlus