
TASIMELTION, an orphan drug for non24
N-([(1R,2R)-2-(2,3-Dihydro-1-benzofuran-4-yl)cyclopropyl]methyl)propanamide
(1R-trans)-N-[[2-(2,3-dihydro-4-benzofuranyl)cyclopropyl]methyl]pro- pananamide VEC162
(-)-(trans)-N-[[2-(2,3-Dihydrobenzofuran-4-yl)cycloprop-1-yl]methyl]propanamide
N-(((1R,2R)-2-(2,3-Dihydro-1-benzofuran-4-yl)cyclopropyl)methyl)propanamide
Bristol-Myers Squibb Company
PRODUCT PATENT
U.S. Pat. No. 5,856,529
January 31, 2014 — The U.S. Food and Drug Administration today approved Hetlioz (tasimelteon), a melatonin receptor agonist, to treat non-24- hour sleep-wake disorder (“non-24”) in totally blind individuals. Non-24 is a chronic circadian rhythm (body clock) disorder in the blind that causes problems with the timing of sleep. This is the first FDA approval of a treatment for the disorder.
Non-24 occurs in persons who are completely blind. Light does not enter their eyes and they cannot synchronize their body clock to the 24-hour light-dark cycle.
http://www.drugs.com/newdrugs/fda-approves-hetlioz-first-non-24-hour-sleep-wake-disorder-blind-individuals-4005.html
VEC-162, BMS-214778, 609799-22-6, Hetlioz, Tasimelteon (USAN/INN), Tasimelteon [USAN:INN], UNII-SHS4PU80D9,
Tasimelteon 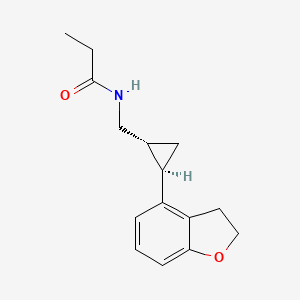
TASIMELTION , BMS-214,778) is a drug which is under development for the treatment of insomnia and other sleep disorders.[1] It is a selective agonistfor the melatonin receptors MT1 and MT2 in the suprachiasmatic nucleus of the brain, similar to older drugs such as ramelteon.[2] It has been through Phase III trials successfully and was shown to improve both onset and maintenance of sleep, with few side effects.[3]
A year-long (2011-2012) study at Harvard is testing the use of tasimelteon in blind subjects with non-24-hour sleep–wake disorder.[4] In May 2013Vanda Pharmaceuticals submitted a New Drug Application to the Food and Drug Administration for Tasimelteon for the treatment of non-24-hour sleep–wake disorder in totally blind people.[5]
SEQUENCE
Discovered by Bristol-Myers Squibb (BMS) and co-developed with Vanda Pharmaceuticals, tasimelteon is a hypnotic family benzofuran. In Phase III development, it has an orphan drug status.
JAN2014.. APPROVED FDA
In mid-November 2013 the FDA announced their recommendation for the approval of Tasimelteon for the treatment of non-24-disorder.Tasimelteon effectively resets the circadian rhythm, helping to restore normal sleep patterns.http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM374388.pdf
January 2010: FDA granted orphan drug tasimelteon to disturbed sleep / wake in blind without light perception.
February 2008: Vanda has completed enrollment in its Phase III trial in chronic primary insomnia.
June 2007: Results of a Phase III trial for transient insomnia tasimelteon presented by Vanda at the 21st annual meeting of the Associated Professional Sleep Societies. These results demonstrated improvements in objective and subjective measures of sleep and its maintenance.
2004 Vanda gets a license tasimelteon (or BMS-214778 and VEC-162) from Bristol-Myers Squibb.
About Tasimelteon: Tasimelteon is a circadian regulator in development for the treatment of Non-24. Tasimelteon is a dual melatonin receptor agonist (DMRA) with selective agonist activityat the MT1 and MT2 receptors.Tasimelteon’s ability to reset the master body clock in the suprachiasmatic nucleus (SCN) results in the entrainment of the body’s melatonin and cortisol rhythms with the 24-hour day-night cycle. The patent claiming tasimelteon as a new chemical entity extends through December 2022, assuming a 5-year extension to be granted under the Hatch-Waxman Act. Tasimelteon has been granted orphan drug designation for the treatment of Non-24 from both the U.S. and the European Union.
Previously, BMS-214778, identified as an agonist of melatonin receptors, has been the subject of pre-clinical studies for the treatment of sleep disorders resulting from a disturbance of circadian rhythms.The first Pharmacokinetic studies were performed in rats and monkeys.
The master body clock controls the timing of many aspects of physiology, behavior and metabolism that show daily rhythms, including the sleep-wake cycles, body temperature, alertness and performance, metabolic rhythms and certain hormones which exhibit circadian variation. Outputs from the suprachiasmatic nucleus (SCN) control many endocrine rhythms including those of melatonin secretion by the pineal gland as well as the control of cortisol secretion via effects on the hypothalamus, the pituitary and the adrenal glands.
This master body clock, located in the SCN, spontaneously generates rhythms of approximately 24.5 hours. These non-24-hour rhythms are synchronized each day to the 24-hour day-night cycle by light, the primary environmental time cue which is detected by specialized cells in the retina and transmitted to the SCN via the retino-hypothalamic tract. Inability to detect this light signal, as occurs in most totally blind individuals, leads to the inability of the master body clock to be reset daily and maintain entrainment to a 24-hour day.
Non-24-Hour Disorder
Non-24, also referred to as Non-24-Hour Sleep-Wake Disorder (N24HSWD) or Non-24-Hour Disorder, is an orphan indication affecting approximately 65,000 to 95,000 people in the U.S. and 140,000 in Europe. Non-24 occurs when individuals, primarily blind with no light perception, are unable to synchronize their endogenous circadian pacemaker to the 24-hour light/dark cycle. Without light as a synchronizer, and because the period of the internal clock is typically a little longer than 24 hours, individuals with Non-24 experience their circadian drive to initiate sleep drifting later and later each day. Individuals with Non-24 have abnormal night sleep patterns, accompanied by difficulty staying awake during the day. Non-24 leads to significant impairment, with chronic effects impacting the social and occupational functioning of these individuals.
In addition to problems sleeping at the desired time, individuals with Non-24 experience excessive daytime sleepiness that often results in daytime napping. TASIMELTION
TASIMELTION
The severity of nighttime sleep complaints and/or daytime sleepiness complaints varies depending on where in the cycle the individual’s body clock is with respect to their social, work, or sleep schedule. The “free running” of the clock results in approximately a 1-4 month repeating cycle, the circadian cycle, where the circadian drive to initiate sleep continually shifts a little each day (about 15 minutes on average) until the cycle repeats itself. Initially, when the circadian cycle becomes desynchronous with the 24 h day-night cycle, individuals with Non-24 have difficulty initiating sleep. As time progresses, the internal circadian rhythms of these individuals becomes 180 degrees out of synchrony with the 24 h day-night cycle, which gradually makes sleeping at night virtually impossible, and leads to extreme sleepiness during daytime hours.
Eventually, the individual’s sleep-wake cycle becomes aligned with the night, and “free-running” individuals are able to sleep well during a conventional or socially acceptable time. However, the alignment between the internal circadian rhythm and the 24-hour day-night cycle is only temporary. In addition to cyclical nighttime sleep and daytime sleepiness problems, this condition can cause deleterious daily shifts in body temperature and hormone secretion, may cause metabolic disruption and is sometimes associated with depressive symptoms and mood disorders.
It is estimated that 50-75% of totally blind people in the United States (approximately 65,000 to 95,000) have Non-24. This condition can also affect sighted people. However, cases are rarely reported in this population, and the true rate of Non-24 in the general population is not known.
The ultimate treatment goal for individuals with Non-24 is to entrain or synchronize their circadian rhythms into an appropriate phase relationship with the 24-hour day so that they will have increased sleepiness during the night and increased wakefulness during the daytime.
INTRODUCTION
Tasimelteon has the chemical name: trans-N-[[2-(2,3-dihydrobenzofuran-4-yl)cycloprop-1yl]methyl]propanamide, has the structure of Formula I:
and is disclosed in U.S. Pat. No. 5,856,529 and in US 20090105333, both of which are incorporated herein by reference as though fully set forth.
Tasimelteon is a white to off-white powder with a melting point of about 78° C. (DSC) and is very soluble or freely soluble in 95% ethanol, methanol, acetonitrile, ethyl acetate, isopropanol, polyethylene glycols (PEG-300 and PEG-400), and only slightly soluble in water. The native pH of a saturated solution of tasimelteon in water is 8.5 and its aqueous solubility is practically unaffected by pH. Tasimelteon has 2-4 times greater affinity for MT2R relative to MT1R. It’s affinity (Ki) for MT1R is 0.3 to 0.4 and for MT2R, 0.1 to 0.2. Tasimelteon is useful in the practice of this invention because it is a melatonin agonist that has been demonstrated, among other activities, to entrain patients suffering from Non-24.
………………………..
SYNTHESIS
(1R-trans)-N-[[2 – (2,3-dihydro-4 benzofuranyl) cyclopropyl] methyl] propanamide PATENT: BRISTOL-MYERS SQUIBB PRIORITY DATE: 1996 HYPNOTIC

PREPARATION OF XV
XXIV D-camphorsulfonic acid IS REACTED WITH THIONYL CHLORIDE TO GIVE
…………XXV (1S, 4R) -7,7-dimethyl-2-oxo-bicyclo [2.2.1] heptane-1-methanesulfonyl chloride
TREATED WITH
XXVI ammonium hydroxide
TO GIVE
XXVII (1S, 4R) -7,7-dimethyl-2-oxo-bicyclo [2.2.1] heptane-1-methanesulfonamide
TREATED WITH AMBERLYST15
….XXVIII (3aS, 6R) -4,5,6,7-tetrahydro-8 ,8-dimethyl-3H-3a ,6-methano-2 ,1-benzisothiazole-2 ,2-dioxide
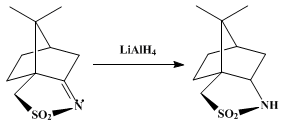
TREATED WITH LAH, ie double bond is reduced to get
…..XV (3aS, 6R, 7aR)-hexahydro-8 ,8-dimethyl-3H-3a ,6-methano-2 ,1-benzisothiazole-2 ,2-dioxide
Intermediate
I 3-hydroxybenzoic acid methyl ester
II 3-bromo-1-propene
III 3 – (2-propenyloxy) benzoic acid methyl ester
IV 3-hydroxy-2-(2-propenyl) benzoic acid methyl ester
V 2,3-dihydro-4-hydroxy-2-benzofurancarboxylic acid methyl ester
VI benzofuran-4-carboxylic acid methyl ester
VII benzofuran-4-carboxylic acid
VIII 2,3-dihydro-4-benzofurancarboxylic acid
IX 2,3-dihydro-4-benzofuranmethanol
X 2,3-dihydro-4-benzofurancarboxaldehyde
XI Propanedioic acid
XII (E) -3 – (2,3-dihydro-4-benzofuranyl) propenoic acid
XIII thionyl chloride
XIV (E) -3 – (2,3-dihydro-4-benzofuranyl) propenoyl chloride
XV (3aS, 6R, 7aR)-hexahydro-8 ,8-dimethyl-3H-3a ,6-methano-2 ,1-benzisothiazole-2 ,2-dioxide
XVI (3aS,6R,7aR)-1-[(E)-3-(2,3-dihydro-4-benzofuranyl)-1-oxo-2-propenyl]hexahydro-8,8-dimethyl-3H-3a,6-methano-2,1-benzisothiazole-2,2-dioxide
XVII (3aS,6R,7aR)-1-[[(1R,2R)-2-(2,3-dihydro-4-benzofuranyl)cyclopropyl]carbonyl]hexahydro-8,8-dimethyl-3H-3a,6-methano-2,1-benzisothiazole-2,2-dioxide
XVIII [R-(R *, R *)] -2 – (2,3-dihydro-4-benzofuranyl) cyclopropanemethanol
XIX [R-(R *, R *)] -2 – (2,3-dihydro-4-benzofuranyl) cyclopropanecarboxaldehyde
XX hydroxylamine hydrochloride
XXI [R-(R *, R *)] -2 – (2,3-dihydro-4-benzofuranyl) cyclopropanecarbaldehyde oxime
XXII [R-(R *, R *)] -2 – (2,3-dihydro-4-benzofuranyl) cyclopropanemethanamine
XXIII propanoyl chloride
XXIV D-camphorsulfonic acid
XXV (1S, 4R) -7,7-dimethyl-2-oxo-bicyclo [2.2.1] heptane-1-methanesulfonyl chloride
XXVI ammonium hydroxide
XXVII (1S, 4R) -7,7-dimethyl-2-oxo-bicyclo [2.2.1] heptane-1-methanesulfonamide
XXVIII (3aS, 6R) -4,5,6,7-tetrahydro-8 ,8-dimethyl-3H-3a ,6-methano-2 ,1-benzisothiazole-2 ,2-dioxide
Bibliography
– Patents: Benzofuran and dihydrobenzofuran melatonergic agents: US5856529 (1999)
Priority: US19960032689P, 10 Dec. 1996 (Bristol-Myers Squibb Company, U.S.)
– Preparation III (quinazolines): US2004044015 (2004) Priority: EP20000402845, 13 Oct. 2000
– Preparation of VII (aminoalkylindols): Structure-Activity Relationships of Novel Cannabinoid Mimetics Eissenstat et al, J.. Med. Chem. 1995, 38, 3094-3105
– Preparation XXVIII: Towson et al. Organic Syntheses, Coll. Vol. 8, p.104 (1993) Vol. 69, p.158 (1990)
– Preparation XV: Weismiller et al. Organic Syntheses, Coll. Vol. 8, p.110 (1993) Vol. 69, p.154 (1990).
– G. Birznieks et al. Melatonin agonist VEC-162 Improves sleep onset and maintenance in a model of transient insomnia. Sleep 2007, 30, 0773 Abstract.
-. Rajaratnam SM et al, The melatonin agonist VEC-162 Phase time immediately advances the human circadian system, Sleep 2006, 29, 0159 Abstract.
-. AK Singh et al, Evolution of a manufacturing route for a highly potent drug candidate, 229th ACS Natl Meet, March 13-17, 2005, San Diego, Abstract MEDI 576.
– Vachharajani NN et al, Preclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonist, J Pharm Sci. 2003 Apr; 92 (4) :760-72.
. – JW Scott et al, Catalytic Asymmetric Synthesis of a melotonin antagonist; synthesis and process optimization. 223rd ACS Natl Meet, April 7-11, Orlando, 2002, Abstract ORGN 186.
…………………….
SYNTHESIS CONSTRUCTION AS IN PATENT
WO1998025606A1
GENERAL SCHEMES
Reaction Scheme 1
The syntheses of the 4-aryl-propenoic acid derivatives, 2 and 3, are shown in Reaction Scheme 1. The starting aldehydes, 1 , can be prepared by methods well known to those skilled in the art. Condensation of malonic acid with the aldehydes, 1, in solvents such as pyridine with catalysts such as piperidine or pyrrolidine, gives the 4-aryl- propenoic acid, 2. Subsequent conversion of the acid to the acid chloride using reagents such as thionyl chloride, phosphoryl chloride, or the like, followed by reaction with N,0-dimethyl hydroxylamine gives the amide intermediate 3 in good yields. Alternatively, aldehyde 1 can be converted directly to amide 3 using reagents such as diethyl (N-methoxy- N-methyl-carbamoylmethyl)phosphonate with a strong base such as sodium hydride.
Reaction Scheme 2
The conversion of the amide intermediate 3 to the racemic, trans- cyclopropane carboxaldehyde intermediate, 4, is shown in Reaction Scheme 2. Intermediate 3 was allowed to react with cyclopropanating reagents such as trimethylsulfoxonium iodide and sodium hydride in solvents such as DMF, THF, or the like. Subsequent reduction using reagents such as LAH in solvents such as THF, ethyl ether, or the like, gives the racemic, trans-cyclopropane carboxaldehyde intermediates, 4.
Reaction Scheme 3
Racemic cyclopropane intermediate 5 (R = halogen) can be prepared from intermediate 2 as shown in Reaction Scheme 3. Intermediate 2 was converted to the corresponding allylic alcohol by treatment with reducing agents such as sodium borohydride plus iodine in solvents such as THF. Subsequent acylation using reagents such as acetic anhydride in pyridine or acetyl chloride gave the allylic acetate which was allowed to react with cyclopropanating reagents such as sodium chloro-difluoroacetate in diglyme to provide the racemic, trans- cyclopropane acetate intermediates, 5. Reaction Scheme 4

The conversion of the acid 2 to the chiral cyclopropane carboxaldehyde intermediate, (-)-(trans)-4, is shown in Reaction Scheme 4. Intermediate 2 is condensed with (-)-2,10-camphorsultam under standard conditions, and then cyclopropanated in the presence of catalysts such as palladium acetate using diazomethane generated from reagents such as 1-methyl-3-nitro-1-nitrosoguanidine. Subsequent reduction using reagents such as LAH in solvents such as THF, followed by oxidation of the alcohol intermediates using reagents such as DMSO/oxalyl chloride, or PCC, gives the cyclopropane carboxaldehyde intermediate, (-)-(trans)-4, in good yields. The enantiomer, (+)-(trans)-4, can also be obtained employing a similar procedure using (+)-2,10- camphorsultam in place of (-)-2,10-camphorsultam.
When it is desired to prepare compounds of Formula I wherein m = 2, the alcohol intermediate may be activated in the conventional manner such as with mesyl chloride and treated with sodium cyanide followed by reduction of the nitrile group with a reducing agent such as LAH to produce the amine intermediate 6.
Reaction Scheme 5


Reaction Scheme 5 shows the conversion of intermediates 4 and 5 to the amine intermediate, 7, and the subsequent conversion of 6. or 7 to compounds of Formula I. The carboxaldehyde intermediate, 4, is condensed with hydroxylamine and then reduced with reagents such as LAH to give the amine intermediate, 7. The acetate intermediate 5 is hydrolyzed with potassium hydroxide to the alcohol, converted to the mesylate with methane sulfonyl chloride and triethyl amine in CH2CI2and then converted to the azide by treatment with sodium azide in solvents such as DMF. Subsequent reduction of the azide group with a reducing agent such as LAH produced the amine intermediate 7. Further reaction of 6 or 7 with acylating reagents gives compounds of Formula I. Suitable acylating agents include carboxylic acid halides, anhydrides, acyl imidazoles, alkyl isocyanates, alkyl isothiocyanates, and carboxylic acids in the presence of condensing agents, such as carbonyl imidazole, carbodiimides, and the like. Reaction Scheme 6

Reaction Scheme 6 shows the alkylation of secondary amides of Formula I (R2 = H) to give tertiary amides of Formula I (R2 = alkyl). The secondary amide is reacted with a base such as sodium hydride, potassium tert-butoxide, or the like, and then reacted with an alkylating reagent such as alkyl halides, alkyl sulfonate esters, or the like to produce tertiary amides of Formula I.
Reaction Scheme 7
Reaction Scheme 7 shows the halogenation of compounds of Formula I. The carboxamides, i (Q1 = Q2 = H), are reacted with excess amounts of halogenating agents such as iodine, N-bromosuccinimide, or the like to give the dihalo-compounds of Formula I (Q1 = Q2 = halogen). Alternatively, a stoichiometric amount of these halogenating agents can be used to give the monohalo-compounds of Formula I (Q1 = H, Q2 = halogen; or Q1 = halogen, Q2 = H). In both cases, additives such as lead IV tetraacetate can be used to facilitate the reaction. Biological Activity of the Compounds
The compounds of the invention are melatonergic agents. They have been found to bind human melatonergic receptors expressed in a stable cell line with good affinity. Further, the compounds are agonists as determined by their ability, like melatonin, to block the forskolin- stimulated accumulation of cAMP in certain cells. Due to these properties, the compounds and compositions of the invention should be useful as sedatives, chronobiotic agents, anxiolytics, antipsychotics, analgesics, and the like. Specifically, these agents should find use in the treatment of stress, sleep disorders, seasonal depression, appetite regulation, shifts in circadian cycles, melancholia, benign prostatic hyperplasia and related conditions
EXPERIMENTAL PROCEDURES
SEE ORIGINAL PATENT FOR CORECTIONS
Preparation 1
Benzofuran-4-carboxaldehyde
Step 1 : N-Methoxy-N-methyl-benzofuran-4-carboxamide
A mixture of benzofuran-4-carboxylic acid [Eissenstat, et al.. J. Medicinal Chemistry, 38 (16) 3094-3105 (1995)] (2.8 g, 17.4 mmol) and thionyl chloride (25 mL) was heated to reflux for 2 h and then concentrated in vacuo. The solid residue was dissolved in ethyl acetate (50 mL) and a solution of N,O-dimethylhydroxylamine hydrochloride (2.8 g) in saturated NaHC03(60 mL) was added with stirring. After stirring for 1.5 h, the ethyl acetate layer was separated. The aqueous layer was extracted with ethyl acetate. The ethyl acetate extracts were combined, washed with saturated NaHCO3 and concentrated in vacuo to give an oil (3.2 g, 95.4%).
Step 2: Benzofuran-4-carboxaldehyde
A solution of N-methoxy-N-methyl-benzofuran-4-carboxamide (3.2 g, 16.6 mmol) in THF (100 mL) was cooled to -45°C and then LAH (0.7 g, 18.7 mmol) was added. The mixture was stirred for 15 min, allowed to warm to -5°C, and then recooled to -45°C. Saturated KHS04 (25 mL) was added with vigorous stirring, and the mixture was allowed to warm to room temperature. The precipitate was filtered and washed with acetone. The filtrate was concentrated in vacuo to give an oil (2.3 g, 94%). Preparation 2
2,3-Dihydrobenzofuran-4-carboxaldehyde
Step 1 : 2,3-Dihydrobenzofuran-4-carboxylic acid
Benzofuran-4-carboxylic acid (10.0 g, 61 .7 mmol) was hydrogenated (60 psi) in acetic acid (100 mL) over 10% Pd/C (2 g) for 12 hr. The mixture was filtered and the filtrate was diluted with water (500 mL) to give 2,3- dihydrobenzofuran-4-carboxylic acid as a white powder (8.4 g, 83%). A sample was recrystallized from isopropanol to give fine white needles (mp: 185.5-187.5°C).
Step 2: (2,3-Dihydrobenzofuran-4-yl)methanol
A solution of 2,3-dihydrobenzofuran-4-carboxylic acid (10 g, 61 mmol) in THF (100 mL) was stirred as LAH (4.64 g, 122 mmol) was slowly added. The mixture was heated to reflux for 30 min. The mixture was cooled and quenched cautiously with ethyl acetate and then with 1 N HCI (150 mL). The mixture was then made acidic with 12 N HCI until all the inorganic precipitate dissolved. The organic layer was separated, and the inorganic layer was extracted twice with ethyl acetate. The organic layers were combined, washed twice with brine, and then concentrated in vacuo. This oil was Kϋgelrohr distilled to a clear oil that crystallized upon cooling (8.53 g, 87.6%).
Step 3: 2.3-Dihydrobenzofuran-4-carboxaldehyde
DMSO (8.10 mL, 1 14 mmol) was added at -78°C to a stirred solution of oxalyl chloride in CH2CI2 (40 mL of a 2M solution). A solution of (2,3- dihydrobenzofuran-4-yl)methanol (8.53 g, 56.9 mmol) in CH2CI2 (35 mL) was added dropwise, and the solution stirred at -78°C for 30 min. Triethyl amine (33 mL, 228 mmol) was added cautiously to quench the reaction. The resulting suspension was stirred at room temperature for 30 min and diluted with CH2CI2 (100 mL). The organic layer was washed three times with water, and twice with brine, and then concentrated in vacuo to an oil (8.42 g, 100%) that was used without purification.
Preparation 16
(±)-(trans)-2-(2,3-Dihyd robenzofuran-4-yl)cyclopropane- carboxaldehyde
Step 1 : (±Htrans)-N-Methoxy-N-methyl-2-(2.3-dihydrobenzofuran-4- yhcyclopropanecarboxamide
Trimethylsulfoxonium iodide (9.9 g, 45 mmol) was added in small portions to a suspension of sodium hydride (1 .8 g, 45 mmol) in DMF (120 mL). After the foaming had subsided (10 min), a solution of (trans)- N-methoxy-N-methyl-3-(2,3-dihydrobenzofuran-4-yl)propenamide (3.5 g, 15 mmol) in DMF (60 mL) was added dropwise, with the temperature maintained between 35-40°C. The mixture was stirred for 3 h at room temperature. Saturated NH4CI (50 mL) was added dropwise and the mixture was extracted three times with ethyl acetate. The organic extracts were combined, washed with H2O and brine, dried over K2CO3, and concentrated in vacuo to give a white wax (3.7 g, 100%).
Step 2: (±)-(trans)- 2-(2.3-Dihydrobenzofuran-4-yl)cyclopropane- carboxaldehyde
A solution of (±)-(trans)-N-methoxy-N-methyl-2-(2,3-dihydrobenzofuran- 4-yl)cyclopropanecarboxamide (3.7 g, 15 mmol) in THF (10 mL) was added dropwise to a rapidly stirred suspension of LAH (683 mg, 18 mmol) in THF (50 mL) at -45°C, maintaining the temperature below -40°C throughout. The cooling bath was removed, the reaction was allowed to warm to 5°C, and then the reaction was immediately recooled to -45°C. Potassium hydrogen sulfate (3.4 g, 25.5 mmol) in H20 (50 mL) was cautiously added dropwise, the temperature maintained below – 30°C throughout. The cooling bath was removed and the suspension was stirred at room temperature for 30 min. The mixture was filtered through Celite and the filter cake was washed with ether. The combined filtrates were then washed with cold 1 N HCI, 1 N NaOH, and brine. The filtrates were dried over MgSO4, and concentrated in vacuo to give a clear oil (2.6 g, 99%).
Preparation 18
(-)-(trans)-2-(2.3-Dihydrobenzofuran-4-yl)cyclopropane-carboxaldehyde
Step 1 : (-Htrans)-N-[3-(2.3-Dihvdrobenzofuran-4-yl)-propenoyll-2.10- camphorsultam
To a solution of (-)-2,10-camphorsultam (8.15 g, 37.9 mmol) in 50 mL toluene at 0°C was added sodium hydride (1.67 g, 41.7 mmol). After stirring for 0.33 h at 0°C and 0.5 h at 20°C and recooling to 0°C, a solution of 3-(2,3-dihydrobenzofuran-4-yl)-2-propenoyl chloride
(37.9 mmol), prepared in situ from the corresponding acid and thionyl chloride (75 mL), in toluene (50 mL), was added dropwise. After stirring for 18 h at 20°C, the mixture was diluted with ethyl acetate and washed with water, 1 N HCI, and 1 N NaOH. The organic solution was dried and concentrated in vacuo to give 15.8 g of crude product. Recrystallization form ethanol-methanol (600 mL, 1 :1) gave the product (13.5 g, 92%, mp 199.5-200°C).
Step 2: (-)-N-[[(trans)-2-(2,3-Dihydrobenzofuran-4-yl)-cyclopropylj- carbonylj-2, 10-camphorsultam
1 -Methyl-3-nitro-1 -nitrosoguanidine (23.88g 163 mmol) was added in portions to a mixture of 10 N sodium hydroxide (60 mL) and ether (200 mL) at 0°C. The mixture was shaken vigorously for 0.25 h and the ether layer carefully decanted into a solution of (-)-N-[3-(2,3-dihydrobenzofuran-4-yl)-2-propenoyl]-2,10-camphorsultam (9.67 g, 25 mmol) and palladium acetate (35 mg) in methylene chloride (200 mL). After stirring for 18 h, acetic acid (5 mL) was added to the reaction and the mixture stirred for 0.5 h. The mixture was washed with 1 N HCI, 1 N NaOH and brine. The solution was dried, concentrated in vacuo and the residue crystallized twice from ethanol to give the product (6.67 g, 66.5%, mp 157-159°C).
Step 3: (-)-(trans)-2-(2,3-Dihydrobenzofuran-4-yl)cyclopropane- methanol
A solution of (-)-N-[(trans)-2-(2,3-dihydrobenzofuran-4-yl)cyclo-propanecarbonylj-2,10-camphorsultam (4.3 g, 10.7 mmol) in THF (50 mL) was added dropwise to a mixture of LAH (0.81 g, 21.4 mmol) in THF (50 mL) at -45°C. The mixture was stirred for 2 hr while it warmed to 10°C. The mixture was recooled to -40°C and hydrolyzed by the addition of saturated KHS0 (20 mL). The mixture was stirred at room temperature for 30 minutes and filtered. The precipitate was washed twice with acetone. The combined filtrate and acetone washes were concentrated in vacuo. The gummy residue was dissolved in ether, washed with 1 N NaOH and 1 N HCI, and then dried in vacuo to give the product (2.0 g, 98.4%).
Step 4: (-)-(trans)-2-(2.3-Dihydrobenzofuran-4-yl)cyclopropane- carboxaldehyde DMSO (1.6 g, 21 mmol) was added to oxalyl chloride in CH2CI2(7.4 mL of 2 M solution, 14.8 mmole) at -78°C. The (-)-(trans)-2-(2,3-dihydrobenzofuran-4-yl)-cyclopropylmethanol (2.0 g, 10.5 mmol) in CH2CI2(15 mL) was added. The mixture was stirred for 20 min and then triethylamine (4.24 g, 42 mmol) was added. The mixture was warmed to room temperature and stirred for 30 min. The mixture was diluted with CH2CI2 and washed with water, 1 N HCI, and then 1 N NaOH. The organic layer was dried and concentrated iι> vacuo to give the aldehyde product (1.98 g, 100%).
Preparation 24
(-)-(trans)-2-(2.3-Dihydrobenzofuran-4-yl)cyclopropane-methanamine A mixture of (-)-(trans)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropane-carboxaldehyde (1.98 g, 10.5 mmol), hydroxylamine hydrochloride (2.29 g, 33 mmol), and 30% NaOH (3.5 mL, 35 mmol), in 5:1
ethanol/water (50 mL) was heated on a steam bath for 2 h. The solution was concentrated in vacuo. and the residue mixed with water. The mixture was extracted with CH2CI2. The organic extracts were dried and concentrated in vacuo to give a solid which NMR analysis showed to be a mixture of the cis and trans oximes. This material was dissolved in THF (20 mL) and added to solution of alane in THF [prepared from LAH (1.14 g, 30 mmol) and H2S04 (1.47 g, 15 mmol) at 0°Cj. The reaction was stirred for 18 h, and quenched successively with water (1.15 mL), 15% NaOH (1.15 mL), and then water (3.45 mL). The mixture was filtered and the filtrate was concentrated in vacuo. The residue was mixed with ether and washed with water and then 1 N HCI. The acid washes were made basic and extracted with CH2CI . The extracts were dried and concentrated in vacuo to give the amine product (1.4 g, 70.5%). The amine was converted to the fumarate salt in ethanol (mp: 197-198°C).
Anal. Calc’d for C12H15NO • C4H404: C, 62.94; H, 6.27; N, 4.59.
Found: C, 62.87; H, 6.31 ; N, 4.52.
FINAL PRODUCT TASIMELTEON
Example 2
(-)-(trans)-N-[[2-(2,3-Dihydrobenzofuran-4-yl)cycloprop-1-yl]methyl]propanamide
This compound was prepared similar to the above procedure using propionyl chloride and (-)-(trans)-2-(2,3-dihydrobenzofuran-4-yl)- cyclopropanemethanamine to give an oil that solidified upon standing to an off-white solid (61 %, mp: 71-72°C). IR (NaCI Film): 3298, 1645, 1548, 1459, 1235 cm“1.
Mo5 : -17.3°
Anal. Calc’d for C15H19N02: C, 73.44; H, 7.87; N, 5.71 . Found: C, 73.28; H, 7.68; N, 5.58.
References
- ‘Time-bending drug’ for jet lag. BBC News. 2 December 2008
- Vachharajani, Nimish N., Yeleswaram, Krishnaswamy, Boulton, David W. (April 2003). “Preclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonist”. Journal of Pharmaceutical Sciences 92 (4): 760–72. doi:10.1002/jps.10348. PMID 12661062.
- Shantha MW Rajaratnam, Mihael H Polymeropoulos, Dennis M Fisher, Thomas Roth, Christin Scott, Gunther Birznieks, Elizabeth B Klerman (2009-02-07). “Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials”. The Lancet 373 (9662): 482–491. doi:10.1016/S0140-6736(08)61812-7. PMID 19054552. Retrieved 2010-02-23.
- Audio interview with Joseph Hull of Harvard, spring 2011
- Vanda Pharmaceuticals seeks FDA approval
- Recent progress in the development of agonists and antagonists for melatonin receptors.Zlotos DP.
 TASIMELTION
TASIMELTION
PATENTS
| US2010261786 |
10-15-2010 |
PREDICTION OF SLEEP PARAMETER AND RESPONSE TO SLEEP-INDUCING COMPOUND BASED ON PER3 VNTR GENOTYPE |
| US2009209638 |
8-21-2009 |
TREATMENT FOR DEPRESSIVE DISORDERS |
| US6060506 |
5-10-2000 |
Benzopyran derivatives as melatonergic agents |
| US5981571 |
11-10-1999 |
Benzodioxa alkylene ethers as melatonergic agents |
| WO9825606 |
6-19-1998 |
BENZODIOXOLE, BENZOFURAN, DIHYDROBENZOFURAN, AND BENZODIOXANE MELATONERGIC AGENTS |
| WO2007137244A1 * |
May 22, 2007 |
Nov 29, 2007 |
Gunther Birznieks |
Melatonin agonist treatment |
| US4880826 |
Jun 25, 1987 |
Nov 14, 1989 |
Nava Zisapel |
Melatonin antagonist |
| US4997845 |
May 10, 1990 |
Mar 5, 1991 |
Eli Lilly And Company |
β-alkylmelatonins as ovulation inhibitors |
| US5093352 |
May 16, 1990 |
Mar 3, 1992 |
Whitby Research, Inc. |
Antidepressant agents |
| US5151446 |
Mar 28, 1991 |
Sep 29, 1992 |
Northwestern University |
Substituted 2-amidotetralins as melatonin agonists and antagonists |
| US5225442 |
Jan 3, 1992 |
Jul 6, 1993 |
Adir Et Compagnie |
Compounds having a naphthalene structure |
| US5580878 |
Jun 7, 1995 |
Dec 3, 1996 |
Interneuron Pharmaceuticals, Inc. |
Substituted tryptamines phenalkylamines and related compounds |
| US5856529 |
Dec 9, 1997 |
Jan 5, 1999 |
Bristol-Myers Squibb Company |
Benzofuran and dihydrobenzofuran melatonergic agents |
| US6211225 |
Jun 6, 2000 |
Apr 3, 2001 |
Bristol-Meyers Squibb |
Heterocyclic aminopyrrolidine derivatives as melatonergic agents |
| US7754902 |
May 18, 2006 |
Jul 13, 2010 |
Vanda Pharmaceuticals, Inc. |
Ruthenium(II) catalysts for use in stereoselective cyclopropanations |
| US20010047016 |
Apr 12, 2001 |
Nov 29, 2001 |
Gregory Oxenkrug |
Method for treating depression |
| US20050164987 |
Dec 22, 2004 |
Jul 28, 2005 |
Barberich Timothy J. |
Melatonin combination therapy for improving sleep quality |
| US20090105333 |
May 22, 2007 |
Apr 23, 2009 |
Gunther Birznieks |
Melatonin agonist treatment |
extra info
Org. Synth. 1990, 69, 154
(−)-D-2,10-CAMPHORSULTAM
[3H-3a,6-Methano-2,1-benzisothiazole, 4,5,6,7-tetrahydro-8,8-dimethyl-2,2-dioxide, (3aS)-]
Submitted by Michael C. Weismiller, James C. Towson, and Franklin A. Davis1.
Checked by David I. Magee and Robert K. Boeckman, Jr..
1. Procedure
(−)-2,10-Camphorsultam. A dry, 2-L, three-necked, round-bottomed flask is equipped with a 1.5-in egg-shaped Teflon stirring bar, a 250-mL addition funnel, and a 300-mL Soxhlet extraction apparatus equipped with a mineral oil bubbler connected to an inert-gas source. The flask is charged with 600 mL of dry tetrahydrofuran (THF) (Note 1) and6.2 g (0.16 mol) of lithium aluminum hydride (Note 2). Into the 50-mL Soxhlet extraction thimble is placed 35.0 g (0.16 mol) of (−)-(camphorsulfonyl)imine (Note 3) and the reaction mixture is stirred and heated at reflux. After all of the(camphorsulfonyl)imine has been siphoned into the reaction flask (3–4 hr), the mixture is allowed to cool to room temperature. The unreacted lithium aluminum hydride is cautiously hydrolyzed by dropwise addition of 200 mL of 1 Nhydrochloric acid via the addition funnel (Note 4). After the hydrolysis is complete the contents of the flask are transferred to a 1-L separatory funnel, the lower, silver-colored aqueous layer is separated, and the upper layer placed in a 1-L Erlenmeyer flask. The aqueous phase is returned to the separatory funnel and washed with methylene chloride (3 × 100 mL). After the reaction flask is rinsed with methylene chloride (50 mL), the organic washings are combined with the THF phase and dried over anhydrous magnesium sulfate for 10–15 min. Filtration through a 300-mL sintered-glass funnel of coarse porosity into a 1-L round-bottomed flask followed by removal of the solvent on arotary evaporator gives 33.5 g (95%) of the crude (−)-2,10-camphorsultam. The crude sultam is placed in a 250-mL Erlenmeyer flask and crystallized from approximately 60 mL of absolute ethanol. The product is collected on a 150-mL sintered-glass funnel of coarse porosity and dried in a vacuum desiccator to give 31.1 g (88%) of the pure sultam. A second crop of crystals can be gained by evaporating approximately half the filtrate; the residue is crystallized as above to give 1.4 g (4%). The combined yield of white crystalline solid, mp 183–184°C, [α]D −30.7° (CHCl3, c 2.3) is92% (Note 5) and (Note 6).
2. Notes
1. Tetrahydrofuran (Aldrich Chemical Company, Inc.) was distilled from sodium benzophenone.
2. Lithium aluminum hydride was purchased from Aldrich Chemical Company, Inc.
3. (−)-(Camphorsulfonyl)imine, [(7S)-(−)-10,10-dimethyl-5-thia-4-azatricyclo[5.2.1.03,7]dec-3-ene 5,5-dioxide] was prepared by the procedure of Towson, Weismiller, Lal, Sheppard, and Davis, Org. Synth., Coll. Vol. VIII, 1993, 104.
4. The addition must be very slow at first (1 drop/5 sec) until the vigorous reaction has subsided.
5. The NMR spectrum of (−)-2,10-camphorsultam is as follows: 1H NMR (CDCl3) δ: 0.94 (s, 3 H, CH3), 1.14 (s, 3 H, CH3), 1.33 (m, 1 H), 1.47 (m,, 1 H), 1.80–2.05 (5 H), 3.09 (d, 1 H, J = 14), 3.14 (d, 1 H, J = 14), 3.43 (m, 1 H), 4.05 (br s, 1 H, NH); 13C NMR (CDCl3) δ: 20.17 (q, CH3), 26.51 (t), 31.55 (t), 35.72 (t), 44.44 (d), 47.15 (s), 50.08 (t), 54.46 (s), 62.48 (d).
6. Checkers obtained material having the same mp (183–184°C) and [α]D − 31.8° (CHCl3, c 2.3).
3. Discussion
(−)-2,10-Camphorsultam was first prepared by the catalytic hydrogenation of (−)-(camphorsulfonyl)imine overRaney nickel.2 Lithium aluminum hydride reduction was used by Oppolzer and co-workers in their synthesis of the sultam.3,4 However, because of the low solubility of the sultam in tetrahydrofuran, a large amount of solvent was required.4 In the procedure described here the amount of solvent is significantly reduced by using a Soxhlet extractor to convey the imine slowly into the reducing medium.5
Oppolzer’s chiral auxiliary,6 (−)-2,10-camphorsultam, is useful in the asymmetric Diels–Alder reaction,3,4 and for the preparation of enantiomerically pure β-substituted carboxylic acids7 and diols,8 in the stereoselective synthesis of Δ2-isoxazolines,9 and in the preparation of N-fluoro-(−)-2,10-camphorsultam, an enantioselective fluorinating reagent.10
References and Notes
- Department of Chemistry, Drexel University, Philadelphia, PA 19104.
- Shriner, R. L.; Shotton, J. A.; Sutherland, H. J. Am. Chem. Soc. 1938, 60, 2794.
- Oppolzer, W.; Chapuis, C.; Bernardinelli, G. Helv. Chim. Acta 1984, 67, 1397.
- Vandewalle, M.; Van der Eycken, J.; Oppolzer, W.; Vullioud, C. Tetrahedron 1986, 42, 4035.
- Davis, F. A.; Towson, J. C.; Weismiller, M. C.; Lal, G.; Carroll,, P. J. J. Am. Chem. Soc. 1988, 110, 8477.
- Oppolzer, W. Tetrahedron 1987, 43, 1969.
- Oppolzer, W.; Mills, R. J.; Pachinger, W.; Stevenson, T. Helv. Chim. Acta 1986, 69, 1542; Oppolzer, W.; Schneider, P. Helv. Chim. Acta 1986, 69, 1817; Oppolzer, W.; Mills, R. J.; Réglier, M. Tetrahedron Lett. 1986, 27, 183; Oppolzer, W.; Poli. G.Tetrahedron Lett. 1986, 27, 4717; Oppolzer, W.; Poli, G.; Starkemann, C.; Bernardinelli, G. Tetrahedron Lett. 1988, 29, 3559.
- Oppolzer, W.; Barras, J-P. Helv. Chim. Acta 1987, 70, 1666.
- Curran, D. P.; Kim, B. H.; Daugherty, J.; Heffner, T. A. Tetrahedron Lett. 1988, 29, 3555.
- Differding, E.; Lang, R. W. Tetrahedron Lett. 1988, 29, 6087.
Org. Synth. 1990, 69, 158
(+)-(2R,8aS)-10-(CAMPHORYLSULFONYL)OXAZIRIDINE
[4H-4A,7-Methanooxazirino[3,2-i][2,1]benzisothiazole, tetrahydro-9,9-dimethyl-, 3,3-dioxide, [4aS-(4aα,7α,8aR*)]]
Submitted by James C. Towson, Michael C. Weismiller, G. Sankar Lal, Aurelia C. Sheppard, Anil Kumar, and Franklin A. Davis
1.
Checked by David I. Magee and Robert K. Boeckman, Jr..
1. Procedure
A. (+)-(1S)-10-Camphorsulfonamide. Into a 2-L, two-necked, round-bottomed flask, equipped with a 250-mL dropping funnel, a magnetic stirring bar, and a reflux condenser fitted with an outlet connected to a disposable pipettedipped in 2 mL of chloroform in a test tube for monitoring gas evolution, were placed 116 g (0.5 mol) ofcamphorsulfonic acid (Note 1) and 750 mL of reagent-grade chloroform. The suspension of camphorsulfonic acid was heated to reflux and 71.4 g (43.77 mL, 0.6 mol, 1.2 equiv) of freshly distilled thionyl chloride was added dropwise over a 1-hr period. Heating was continued until gas evolution (sulfur dioxide and hydrogen chloride) had ceased (approximately 9–10 hr). The resultant solution of camphorsulfonyl chloride in chloroform was converted tocamphorsulfonamide without further purification.
In a 5-L, two-necked, round-bottomed flask fitted with a 250-mL dropping funnel and a mechanical stirrer was placed a solution of 1.6 L of reagent-grade ammonium hydroxide solution and the flask was cooled to 0°C in an ice bath. The solution of the crude camphorsulfonyl chloride, prepared in the preceding section, was added dropwise to the ammonium hydroxide solution at 0–10°C over a period of 1 hr. The reaction mixture was warmed to room temperature, stirred for 4 hr, the organic layer separated, and the aqueous layer was extracted with methylene chloride (3 × 250 mL). The combined organic layers were washed with brine (250 mL) and dried over anhydrousmagnesium sulfate. Removal of the solvent on the rotary evaporator gave 104.0 g (90%) of the crudecamphorsulfonamide (Note 2) and (Note 3).
B. (−)-(Camphorsulfonyl)imine. A 1-L, round-bottomed flask is equipped with a 2-in. egg-shaped magnetic stirring bar, a Dean–Stark water separator, and a double-walled condenser containing a mineral oil bubbler connected to an inert gas source. Into the flask are placed 5 g of Amberlyst 15 ion-exchange resin (Note 4) and 41.5 g of the crude(+)-(1S)-camphorsulfonamide in 500 mL of toluene. The reaction mixture is heated at reflux for 4 hr. After the reaction flask is cooled, but while it is still warm (40–50°C), 200 mL of methylene chloride is slowly added to dissolve any(camphorsulfonyl)imine that crystallizes. The solution is filtered through a 150-mL sintered glass funnel of coarse porosity an the reaction flask and filter funnel are washed with an additional 75 mL of methylene chloride.
Isolation of the (−)-(camphorsulfonyl)imine is accomplished by removal of the toluene on the rotary evaporator. The resulting solid is recrystallized from absolute ethanol (750 mL) to give white crystals, 34.5–36.4 g (90–95%), mp225–228°C; [α]D −32.7° (CHCl3, c 1.9) (Note 5).
C. (+)-(2R, 8aS)-10-Camphorylsulfonyloxaziridine. A 5-L, three-necked, round-bottomed Morton flask is equipped with an efficient mechanical stirrer, a 125-mm Teflon stirring blade, a Safe Lab stirring bearing (Note 6), and a 500-mL addition funnel. Into the flask are placed the toluene solution of (−)-(camphorsulfonyl)imine (39.9 g, 0.187 mol)prepared in Step B and a room-temperature solution of 543 g (3.93 mol, 7 equiv based on oxone) of anhydrouspotassium carbonate dissolved in 750 mL of water. The reaction mixture is stirred vigorously and a solution of 345 g (0.56 mol, 6 equiv of KHSO5) of oxone dissolved in 1250 mL of water is added dropwise in three portions over 45 min(Note 7) and (Note 8). Completion of the oxidation is determined by TLC (Note 9) and the reaction mixture is filtered through a 150-mL sintered-glass funnel of coarse porosity to remove solids. The filtrate is transferred to a 3-L separatory funnel, the toluene phase is separated and the aqueous phase is washed with methylene chloride (3 × 100 mL). The filtered solids and any solids remaining in the Morton flask are washed with an additional 200 mL of methylene chloride. The organic extracts are combined and washed with 100 mL of saturated sodium sulfite, dried over anhydrousmagnesium sulfate for 15–20 min, filtered, and concentrated on the rotary evaporator. The resulting white solid is crystallized from approximately 500 mL of hot 2-propanol to afford, after drying under vacuum in a desiccator, 35.9 g(84%) of white needles, mp 165–167°C, [α]D +44.6° (CHCl3, c 2.2) (Note 10) and (Note 11).
(−)-(2S,8aR)-10-(camphorylsulfonyl)oxaziridine is prepared in a similar manner starting from (−)-10-camphorsulfonic acid; mp 166–167°C, [α]D +43.6° (CHCl3, c 2.2).
2. Notes
1. (1S)-(+)-10-Camphorsulfonic acid was purchased from Aldrich Chemical Company, Inc.
2. The crude sulfonamide is contaminated with 5–10% of the (camphorsulfonyl)imine, the yield of which increases on standing.
3. The 1H NMR spectrum of (+)-(1S)-10-camphorsulfonamide is as follows: (CDCl3) δ: 0.93 (s, 3 H, CH3), 1.07 (s, 3 H, CH3), 1.40–2.50 (m, 7 H), 3.14 and 3.53 (AB quartet, 2 H, CH2-SO2, J = 15.1), 5.54 (br s, 2 H, NH2).
4. Amberlyst 15 ion-exchange resin is a strongly acidic, macroreticular resin purchased from Aldrich Chemical Company, Inc.
5. The spectral properties of (−)-(camphorsulfonyl)imine are as follows: 1H NMR (CDCl3) δ: 1.03 (s, 3 H, CH3), 1.18 (s, 3 H, CH3), 1.45–2.18 (m, 6 H), 2.65 (m, 1 H), 3.10 and 3.28 (AB quartet, 2 H, CH2-SO2, J = 14.0); 13C NMR (CDCl3) δ: 19.01 (q, CH3), 19.45 (q, CH3), 26.64 (t), 28.44 (t), 35.92 (t), 44.64 (d), 48.00 (s), 49.46 (t), 64.52 (s), 195.52 (s); IR (CHCl3) cm−1: 3030, 2967, 1366. Checkers obtained material having identical melting point and [α]D−32.3° (CHCl3, c 1.8).
6. The SafeLab Teflon bearing can be purchased from Aldrich Chemical Company, Inc. A glass stirring bearing lubricated with silicone grease is unsatisfactory because the dissolved salts solidify in the shaft, causing freezing.
7. Efficient stirring is important and indicated by a milky white appearance of the solution.
8. Occasionally batches of oxone purchased from Aldrich Chemical Company, Inc., have exhibited reduced reactivity in this oxidation. Oxone exposed to moisture prior to use also gives reduced reactivity in this oxidation. If this occurs, oxone is added until oxidation is complete as determined by TLC (Note 9). Potassium carbonate is added as needed to maintain the pH at approximately 9.0. Oxone stored in the refrigerator under an inert atmosphere has shown no loss in reactivity for up to 6 months.
9. Oxidation is generally complete after addition of the oxone solution. The oxidation is monitored by TLC as follows. Remove approximately 0.5 mL of the toluene solution from the nonstirring solution, spot a 250-μm TLC silica gel plate, elute with methylene chloride, and develop with 10% molybdophosphoric acid in ethanol and heating(camphorsulfonyl)imine Rf = 0.28 and (camphorylsulfonyl)oxaziridine Rf = 0.62. If (camphorsulfonyl)imine is detected, stirring is continued at room temperature until the reaction is complete (see (Note 8)). If the reaction mixture takes on a brownish color after addition of oxone and has not gone to completion after 30 min, the reaction mixture is filtered through a 150-mL sintered-glass funnel of coarse porosity, and the solids are washed with 50 mL of methylene chloride. The aqueous/organic extracts are returned to the 5-L Morton flask and stirred vigorously and 52 g (0.08 mol, 1 equiv KHSO5) of oxone is added over 5 min and stirring continued until oxidation is complete (approximately 10–15 min).
10. The submitters employed a toluene solution of crude imine prepared in Part B and obtained somewhat higher yields (90–95%). However, the checkers obtained yields in this range on one half the scale using isolatedsulfonylimine.
11. The spectral properties of (+)-(camphorsulfonyl)oxaziridine are as follows: 1H NMR (CDCl3) δ: 1.03 (s, 3 H, CH3), 1.18 (s, 3 H, CH3), 1.45–2.18 (m, 6 H), 2.65 (d, 1 H), 3.10 and 3.28 (AB quartet, 2 H, CH2-SO2, J = 14.0); 13C NMR (CDCl3) δ: 19.45 (q, CH3), 20.42 (q, CH3), 26.55 (t), 28.39 (t), 33.64 (t), 45.78 (d), 48.16 (s), 48.32 (t), 54.07 (s), 98.76 (s). The checkers obtained material (mp 165–167°C) having [α]D +44.7° (CHCl3, c 2.2).
3. Discussion
Camphorsulfonamide, required for the preparation of the (camphorsulfonyl)imine, was previously prepared in two steps. The first step involved conversion of camphorsulfonic acid to the sulfonyl chloride with PCl5 or SOCl2. The isolated sulfonyl chloride was converted in a second step to the sulfonamide by reaction with ammonium hydroxide. This modified procedure is more efficient because it transforms camphorsulfonic acid directly to camphorsulfonamide, avoiding isolation of the camphorsulfonyl chloride.
(Camphorsulfonyl)imine has been reported as a by-product of reactions involving the camphorsulfonamide.2,3,4,5Reychler in 1898 isolated two isomeric camphorsulfonamides,2 one of which was shown to be the(camphorsulfonyl)imine by Armstrong and Lowry in 1902.3 Vandewalle, Van der Eycken, Oppolzer, and Vullioud described the preparation of (camphorsulfonyl)imine in 74% overall yield from 0.42 mol of the camphorsulfonyl chloride.6 The advantage of the procedure described here is that, by using ammonium hydroxide, the camphorsulfonyl chloride is converted to the sulfonamide in >95% yield.7 The sulfonamide is of sufficient purity that it can be used directly in the cyclization step, which, under acidic conditions, is quantitative in less than 4 hr. These modifications result in production of the (camphorsulfonyl)imine in 86% overall yield from the sulfonyl chloride.
In addition to the synthesis of enantiomerically pure (camphorylsulfonyl)oxaziridine7 and its derivatives,8 the(camphorsulfonyl)imine has been used in the preparation of (−)-2,10-camphorsultam (Oppolzers’ auxiliary),6,9 (+)-(3-oxocamphorysulfonyl) oxaziridine,10 and the N-fluoro-2,10-camphorsultam, an enantioselective fluorinating reagent.11
The N-sulfonyloxaziridines are an important class of selective, aprotic oxidizing reagents.12 13 14 Enantiomerically pure N-sulfonyloxaziridines have been used in the asymmetric oxidation of sulfides to sulfoxides (30–91% ee),15selenides to selenoxides (8–9% ee).16 disulfides to thiosulfinates (2–13% ee),5 and in the asymmetric epoxidation of alkenes (19–65% ee).17,18 Oxidation of optically active sulfonimines (R*SO2N=CHAr) affords mixtures of N-sulfonyloxaziridine diastereoisomers requiring separation by crystallization and/or chromatography.3
(+)-(Camphorylsulfonyl)oxaziridine described here is prepared in four steps from inexpensive (1S)-(+)- or (1R)-(+)-10-camphorsulfonic acid in 77% overall yield.7 Separation of the oxaziridine diastereoisomers is not required because oxidation is sterically blocked from the exo face of the C-N double bond in the (camphorsulfonyl)imine. In general, (camphorsulfonyl)oxaziridine exhibits reduced reactivity compared to other N-sulfonyloxaziridines. For example, while sulfides are asymmetrically oxidized to sulfoxides (3–77% ee), this oxaziridine does not react with amines or alkenes.7 However, this oxaziridine is the reagent of choice for the hydroxylation of lithium and Grignard reagents to give alcohols and phenols because yields are good to excellent and side reactions are minimized.19 This reagent has also been used for the stereoselective oxidation of vinyllithiums to enolates.20
The most important synthetic application of the (camphorylsulfonyl)oxaziridines is the asymmetric oxidation of enolates to optically active α-hydroxy carbonyl compounds.14,21,22,23,24 Chiral, nonracemic α-hydroxy carbonylcompounds have been used extensively in asymmetric synthesis, for example, as chiral synthons, chiral auxiliaries, and chiral ligands. This structural array is also featured in many biologically active natural products. This oxidizing reagent gives uniformly high chemical yields regardless of the counterion, and stereoselectivities are good to excellent (50–95% ee).9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 Since the configuration of the oxaziridine three-membered ring controls the stereochemistry, both α-hydroxy carbonyl optical isomers are readily available. Representative examples of the asymmetric oxidation of prochiral enolates by (+)-(2R,8aS)-camphorylsulfonyl)oxaziridine are given in Tables I and II.
This preparation is referenced from:
- Org. Syn. Coll. Vol. 8, 110
- Org. Syn. Coll. Vol. 9, 212
-
References and Notes
- Department of Chemistry, Drexel University, Philadelphia, PA 19104.
- Reychler, M. A. Bull. Soc. Chim. III 1889, 19, 120.
- Armstrong, H. E.; Lowry, T. M. J. Chem. Soc., Trans. 1902, 81, 1441.
- Dauphin, G.; Kergomard, A.; Scarset, A. Bull. Soc. Chim. Fr. 1976, 862.
- Davis, F. A.; Jenkins, Jr., R. H.; Awad, S. B.; Stringer, O. D.; Watson, W. H.; Galloy, J. J. Am. Chem. Soc. 1982, 104, 5412.
- Vandewalle, M.; Van der Eycken, J.; Oppolzer, W.; Vullioud, C. Tetrahedron, 1986, 42, 4035.
- Davis, F. A.; Towson, J. C.; Weismiller, M. C.; Lal, S.; Carroll, P. J. J. Am. Chem. Soc. 1988, 110, 8477.
- Davis, F. A.; Weismiller, M. C.; Lal, G. S.; Chen, B. C.; Przeslawski, R. M. Tetrahedron Lett., 1989, 30, 1613.
- Oppolzer, W. Tetrahedron 1987, 43, 1969.
- Glahsl, G.; Herrmann, R. J. Chem. Soc., Perkin Trans. I 1988, 1753.
- Differding, E.; Lang, R. W. Tetrahedron Lett. 1988, 29, 6087.
- For recent reviews on the chemistry of N-sulfonyloxaziridines, see: (a) Davis, F. A.; Jenkins, Jr., R. H. in “Asymmetric Synthesis,” Morrison, J. D., Ed.; Academic Press: Orlando, FL, 1984, Vol. 4, Chapter 4;
- Davis, F. A.; Haque, S. M. in “Advances in Oxygenated Processes,” Baumstark, A. L., Ed.; JAI Press: London, Vol. 2;
- Davis, F. A.; Sheppard, A. C. Tetrahedron 1989, 45, 5703.
- Davis, F. A.; McCauley, Jr., J. P.; Chattopadhyay, S.; Harakal, M. E.; Towson, J. C.; Watson, W. H.; Tavanaiepour, I. J. Am. Chem. Soc. 1987, 109, 3370.
- Davis, F. A.; Stringer, O. D.; McCauley, Jr., J. M. Tetrahedron 1985, 41, 4747.
- Davis, F. A.; Chattopadhyay, S. Tetrahedron Lett. 1986, 27, 5079.
- Davis, F. A.; Harakal, M. E.; Awad, S. B. J. Am. Chem. Soc. 1983, 105, 3123.
- Davis, F. A.; Wei, J.; Sheppard, A. C.; Gubernick S. Tetrahedron Lett. 1987, 28, 5115.
- Davis, F. A.; Lal, G. S.; Wei, J. Tetrahedron Lett. 1988, 29, 4269.
- Davis, F. A.; Haque, M. S.; Ulatowski, T. G.; Towson, J. C. J. Org. Chem. 1986, 51, 2402.
- Davis, F. A.; Haque, M. S. J. Org. Chem. 1986, 51, 4083; Davis, F. A.; Haque, M. S.; Przeslawski, R. M. J. Org. Chem. 1989, 54, 2021.
- Davis, F. A.; Ulatowski, T. G.; Haque, M. S. J. Org. Chem. 1987, 52, 5288.
- Davis, F. A.; Sheppard, A. C., Lal, G. S. Tetrahedron Lett. 1989, 30, 779.
- Davis, F. A.; Sheppard, A. C.; Chen, B. C.; Haque, M. S. J. Am. Chem. Soc. 1990, 112, 6679.
OLD ARTICLE
Melatonin can be used to treat sleep disorders, but it has poor bioavailability, and has a half-life in the body of only about 15 minutes
The body’s circadian rhythms – the normal variations in physiological parameters during the day – are very closely involved in regulation of sleep patterns. If these rhythms become out of synch, sleep patterns tend to be disrupted. Melatonin, a hormone produced in the pineal gland, is involved in the sleep–wake cycle of the circadian rhythm. It is excreted in response to a cascade of signals resulting from changes in light level, and the level that is present in the bloodstream varies through the day, with its release eventually resulting in the process of falling asleep.
Melatonin can be used to treat sleep disorders, but it has poor bioavailability, and has a half-life in the body of only about 15 minutes. In addition, side-effects can be an issue as it binds non-selectively to many different receptors within the brain. As a result, there has been a degree of interest in analogues, and one, Takeda’s ramelteon (Rozerem) is approved in the US. Another, tasimelteon, is being developed by Vanda Pharmaceuticals, under licence from Bristol-Myers Squibb.1 It acts as a selective agonist at the MT1 and MT2 melatonin receptors in the brain’s suprachiasmic nucleus, which is a group of neurons in the anterior hypothalamus.

Tasimelteon
In a Phase II trial in induced insomnia, 39 healthy subjects were monitored for seven nights – three at baseline, three after a five-hour advance of the sleep-wake cycle with treatment with 10, 20, 50 or 100mg of tasimelteon or placebo before sleep, and a further night after treatment.2 The drug reduced sleep latency, and also increased sleep efficiency compared with placebo, with the shift in plasma melatonin rhythm being dose-dependent.
– See more at:
http://www.manufacturingchemist.com/technical/article_page/Sleep_disorders__tasimelteon/90220
http://www.manufacturingchemist.com/technical/article_page/Sleep_disorders__tasimelteon/90220#sthash.zya38ov4.dpuf



















 Motesanib
Motesanib


 TASIMELTION
TASIMELTION










 TASIMELTION
TASIMELTION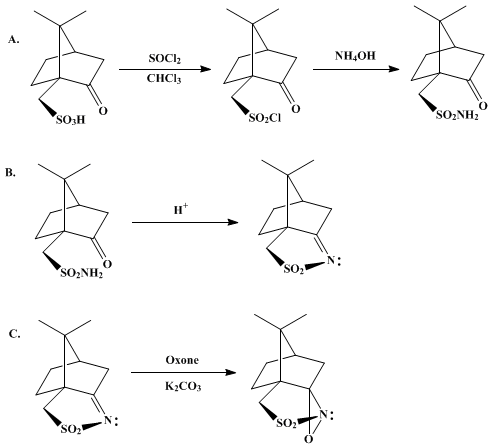
 Tasimelteon
Tasimelteon Empagliflozin
Empagliflozin
 LX4211
LX4211






