Alkermes obtains US FDA fast-track status for depression drug
Irish biotechnology firm Alkermes has received fast-track designation for its proprietary investigational medicine ALKS 5461 from the US Food and Drug Administration (FDA).
Fast-track designation was granted for the adjunctive treatment of patients with major depressive disorder (MDD) who don’t respond to standard therapies. The process intended to speed-up the review of drugs, which treat serious conditions and fill an unmet medical need.
http://www.pharmaceutical-technology.com/news/newsalkermes-obtains-us-fda-fast-track-status-for-depression-drug?WT.mc_id=DN_News
see a presentation
Ehrich ALKS 5461 Presentation
2013 Alkermes. All rights reserved. ALKS 5461: Rethinking. Psychiatry With. Opioid System Modulation. Alkermes R&D Day. July 17, 2013. Elliot Ehrich, M.D..
Patients Tested
Alkermes’s drug was tested in patients for whom other medicines, called selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, haven’t worked as well as hoped. Treatments in those classes include Eli Lilly & Co. (LLY)’s Prozac and Cymbalta. ALKS 5461 was tested on top of drugs patients were already taking as that may be the way the medicine would be used if approved, Pops said.
“This is a potentially very important positive development for Alkermes as ALKS 5461 is an oral, once-a-day drug which has a novel mechanism of action for treating MDD, which represents a very large market opportunity,” Cory Kasimov, an analyst with JPMorgan Chase & Co. (JPM), wrote in a research note today.
About 16.1 million people in the U.S. experience major depressive disorder, or MDD, each year and many don’t get enough of a benefit from the first antidepressants they try, according to Alkermes. Of about 10 million patients who receive treatment for MDD, two-thirds aren’t adequately helped and try a second therapy, Pops said.
ALKS 5461 is a combination of another compound, ALKS 33, and buprenorphine, a therapy that stimulates the opioid system and is approved for treatment of addiction to opioids such as heroin.
The company decided to test the combination based on the premise that opioids have been shown to help in treatment of depression. The problem was their addictive properties, Pops said. ALKS 33 is an opioid receptor blocker, and its use is designed to combat addictive effects.
“We wanted to know if you can decouple the addictive properties of an opioid from antidepressive properties,” Pops said. With ALKS 5461, “we’re pushing the gas and the brake at the same time.”
Alkermes is equipped to start a bigger trial on its own, and may talk with pharmaceutical companies about partnerships to sell the drug outside the U.S. if it’s approved, Pops said, noting “it’s too early” to discuss commercial plans
Sexually Transmitted diseases
Psoriasis
CILNIDIPINE 西尼地平

cilnidipine
西尼地平
CAS 132203-70-4
- (E) – (±) 1 ,4 a dihydro-2 ,6 – dimethyl-4 – (3 – nitrophenyl) -3,5 – pyridinedicarboxylic acid, 2 – methoxy- ethyl butylester 3 – phenyl – 2 – propenyl ester FRC-8653 Cinalong
- More FRC 8653 1,4-Dihydro-2 ,6-dimethyl-4-(3-nitrophenyl) 3 ,5-pyridinedicarboxylic acid 2-methoxyethyl (2E)-3-phenyl-2-propenyl ester
- Molecular formula:C 27 H 28 N 2 O 7
- Molecular Weight:492.52

Cilnidipine (INN) is a calcium channel blocker. It is sold as Atelec in Japan, asCilaheart, Cilacar in India, and under various other trade names in East Asian countries.
Cilnidipine is a dual blocker of L-type voltage-gated calcium channels in vascular smooth muscle and N-type calcium channels in sympathetic nerve terminals that supply blood vessels. However, the clinical benefits of cilnidipine and underlying mechanisms are incompletely understood.
Clinidipine is the novel calcium antagonist accompanied with L-type and N-type calcium channel blocking function. It was jointly developed by Fuji Viscera Pharmaceutical Company, Japan and Ajinomoto, Japan and approved to come into market for the first time and used for high blood pressure treatment in 1995. in india j b chemicals & pharmaceuticals ltd and ncube pharmaceutical develope a market of cilnidipine.
Hypertension is one of the most common cardiovascular disease states, which is defined as a blood pressure greater than or equal to 140/90 mm Hg. Recently, patients with adult disease such as hypertension have rapidly increased. Particularly, since damages due to hypertension may cause acute heart disease or myocardial infarction, etc., there is continued demand for the development of more effective antihypertensive agent.
Meanwhile, antihypertensive agents developed so far can be classified into Angiotensin II Receptor Blocker (ARB), Angiotensin-Converting Enzyme Inhibitor (ACEI) or Calcium Chanel Blocker (CCB) according to the mechanism of actions. Particularly, ARB or CCB drugs manifest more excellent blood pressure lowering effect, and thus they are more frequently used.
However, these drugs have a limit in blood pressure lowering effects, and if each of these drugs is administered in an amount greater than or equal to a specific amount, various side-effects may be caused. Therefore, there have been many attempts in recent years to obtain more excellent blood pressure lowering effect by combination therapy or combined preparation which combines or mixes two or more drugs.
Particularly, since side-effect due to each drug is directly related to the amount or dose of a single drug, there have been active attempts to combine or mix two or more drugs thereby obtaining more excellent blood pressure lowering effect through synergism of the two or more drugs while reducing the amount or dose of each single drug.
For example, US 20040198789 discloses a pharmaceutical composition for lowering blood pressure combining lercanidipine, one of CCB, and valsartan, irbesartan or olmesartan, one of ARB, etc. In addition, a combined preparation composition which combines or mixes various blood pressure lowering drugs or combination therapy thereof has been disclosed.
cilnidipine Compared with other calcium antagonists, clinidipine can act on the N-type calcium-channel that existing sympathetic nerve end besides acting on L-type calcium-channel that similar to most of the calcium antagonists. Due to its N-type calcium-channel blocking properties, it has more advantages compared to conventional calcium-channel blockers. It has lower incidence of Pedal edema, one of the major adverse effects of other calcium channel blockers. Cilnidipine has similar blood pressure lowering efficacy as compared to amlodipine. One of the distinct property of cilnidipine from amlodipine is that it does not cause reflex tachycardia.
In recent years, cardiovascular disease has become common, the incidence increased year by year, about a patient of hypertension in China. 3-1. 500 million, complications caused by hypertension gradually increased, and more and more young patients with hypertension technology. In recent years, antihypertensive drugs also have great development, the main first-line diuretic drug decompression 3 – blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, ar blockers and vascular angiotensin II (Ang II) receptor antagonist.
In the anti-hypertensive drugs, calcium antagonists are following a – blockers after another rapidly developing cardiovascular drugs, has been widely used in clinical hypertension, angina and other diseases, in cardiovascular drugs in the world, ranked first.
Cilnidipine for the long duration of the calcium channel blockers, direct relaxation of vascular smooth muscle, dilation of peripheral arteries, the peripheral resistance decreased, with lower blood pressure, heart rate without causing a reflex effect.
Cilnidipine is a dihydropyridine CCB as well as an antihypertensive. Cilnidipinehas L- and N-calcium channel blocking actions. Though many of the dihydropyridine CCBs may cause an increase in heart rate while being effective for lowering blood pressure, it has been confirmed that cilnidipine does not increase the heart rate and has a stable hypotensive effect. (Takahiro Shiokoshi, “Medical Consultation & New Remedies” vol. 41, No. 6, p. 475-481)
- http://www.mcyy.com.cn/e-product2.asp
- Löhn M, Muzzulini U, Essin K, et al. (May 2002). “Cilnidipine is a novel slow-acting blocker of vascular L-type calcium channels that does not target protein kinase C”. J. Hypertens. 20 (5): 885–93. PMID 12011649.
Cilnidipine (CAS NO.: 132203-70-4), with its systematic name of (+-)-(E)-Cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate, could be produced through many synthetic methods.
Following is one of the synthesis routes: By cyclization of 2-(3-nitrobenzylidene)acetocetic acid cinnamyl ester (I) with 2-aminocrotonic acid 2-methoxyethyl ester (II) by heating at 120 °C.
MORE
NMR
CARBOHYDRATE POLYMERS 90 PG 1719-1724 , YR2012
Numerous peaks were found in the spectrum of cilnidipine: 2.3555 (3H, s, CH3), 2.3886(3H, s, CH3), 3.2843(CD3OD), 3.3292(3H, s, OCH3), 3.5255–3.5623(2H, m, CH3OCH2CH2 ), 4.1224–4.1597(2H, m, CH3OCH2CH2 ), 4.6695–4.7293(2H, m, CH2 CH CH ), 4.8844(D2O), 5.1576(1H, s, CH), 6.2609(1H, dt, CH2 CH CH ), 6.5518(1H, d, CH2 CH CH ), 7.2488–7.3657(6H, m, ArH), 7.7002(1H, dd, ArH), 7.9805(1H, dd, ArH), 8.1548(1H, s, ArH)
References:
Dihydropyridine calcium channel blocker. Prepn: T. Kutsuma et al., EP 161877; eidem, US 4672068(1985, 1987 both to Fujirebio).
Pharmacology: K. Ikeda et al., Oyo Yakuri 44, 433 (1992).
Mechanism of action study: M. Hosonoet al., J. Pharmacobio-Dyn. 15, 547 (1992).
LC-MS determn in plasma: K. Hatada et al., J. Chromatogr. 583, 116 (1992). Clinical study: M. Ishii, Jpn. Pharmacol. Ther. 21, 59 (1993).
Acute toxicity study: S. Wada et al., Yakuri to Chiryo 20, Suppl. 7, S1683 (1992), C.A. 118, 32711 (1992).
U.S Patent No. 4,572,909 discloses amlodipine; U.S Patent No. 4,446,325 discloses aranidipine; U.S Patent No. 4,772,596 discloses azelnidipine; U.S Patent No. 4,220,649 discloses barnidipine; U.S Patent No. 4,448,964 discloses benidipine; U.S Patent No. 5,856,346 discloses clevidipine; U.S Patent No. 4,466,972 discloses isradipine; U.S Patent No. 4,885,284 discloses efonidipine; and U.S Patent No. 4,264,61 1 discloses felodipine.
U.S Patent No. 5,399,578 discloses Valsartan; European Patent No. 0 502 314 discloses Telmisartan; U.S Patent No. 5,138,069 discloses Losartan; U.S Patent No. 5,270,317 discloses Irbesartan; U.S Patent No. 5,583,141 and 5,736,555 discloses Azilsartan; U.S Patent No. 5,196,444 discloses Candesartan; U.S Patent No. 5,616,599 discloses Olmesartan; and U.S Patent No. 5,185,351 discloses Eprosartan.
U.S Patent No. 4,374,829 discloses enalapril; U.S Patent No. 4,587,258 discloses ramipril; U.S Patent No. 4,344,949 discloses quinapril; U.S Patent No. 4,508,729 discloses perindopril; U.S Patent No. 4,374,829 discloses lisinopril; U.S Patent No. 4,410,520 discloses benazepril; U.S Patent No. 4,508,727 discloses imidapril; U.S Patent No. 4,316,906 discloses zofenopril; U.S Patent Nos. 4,046,889 and 4,105,776 discloses captopril; and U.S Patent No. 4,337,201 discloses fosinopril.
-
Amlodipine is 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine disclosed in USP 4,572,909, Japanese patent publication No. Sho 58-167569 and the like.
-
Aranidipine is 3-(2-oxopropoxycarbonyl)-2,6-dimethyl-5-methoxycarbonyl-4-(2-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,446,325 and the like.
-
Azelnidipine is 2-amino-3-(1-diphenylmethyl-3-azetidinyloxycarbonyl)-5-isopropoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,772,596, Japanese patent publication No. Sho 63-253082 and the like.
-
Barnidipine is 3-(1-benzyl-3-pyrrolidinyloxycarbonyl)-2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,220,649, Japanese patent publication No. Sho 55-301 and the like.
-
Benidipine is 3-(1-benzyl-3-piperidinyloxycarbonyl)-2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine and is described in the specifications of U.S. Patent No. 4,501,748, Japanese patent publication No. Sho 59-70667 and the like.
-
Cilnidipine is 2,6-dimethyl-5-(2-methoxyethoxycarbonyl)-4-(3-nitrophenyl)-3-(3-phenyl-2-propenyloxycarbonyl)-1,4-dihydropyridine disclosed in USP 4,672,068, Japanese patent publication No. Sho 60-233058 and the like.
-
Efonidipine is 3-[2-(N-benzyl-N-phenylamino)ethoxycarbonyl]-2,6-dimethyl-5-(5,5-dimethyl-1,3,2-dioxa-2-phosphonyl)-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,885,284, Japanese patent publication No. Sho 60-69089 and the like.
-
Elgodipine is 2,6-dimethyl-5-isopropoxycarbonyl-4-(2,3-methylenedioxyphenyl)-3-[2-[N-methyl-N-(4-fluorophenylmethyl)amino]ethoxycarbonyl]-1,4-dihydropyridine disclosed in USP 4,952,592, Japanese patent publication No. Hei 1-294675 and the like.
-
Felodipine is 3-ethoxycarbonyl-4-(2,3-dichlorophenyl)-2,6-dimethyl-5-methoxycarbonyl-1,4-dihydropyridine disclosed in USP 4,264,611, Japanese patent publication No. Sho 55-9083 and the like.
-
Falnidipine is 2,6-dimethyl-5-methoxycarbonyl-4-(2-nitrophenyl)-3-(2-tetrahydrofurylmethoxycarbonyl)-1,4-dihydropyridine disclosed in USP 4,656,181, Japanese patent publication (kohyo) No. Sho 60-500255 and the like.
-
Lemildipine is 2-carbamoyloxymethyl-4-(2,3-dichlorophenyl)-3-isopropoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine disclosed in Japanese patent publication No. Sho 59-152373 and the like.
-
Manidipine is 2,6-dimethyl-3-[2-(4-diphenylmethyl-1-piperazinyl)ethoxycarbonyl]-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,892,875, Japanese patent publication No. Sho 58-201765 and the like.
-
Nicardipine is 2,6-dimethyl-3-[2-(N-benzyl-N-methylamino)ethoxycarbonyl]-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 3,985,758, Japanese patent publication No. Sho 49-108082 and the like.
-
Nifedipine is 2,6-dimethyl-3,5-dimethoxycarbonyl-4-(2-nitrophenyl)-1,4-dihydropyridine disclosed in USP 3,485,847 and the like.
-
Nilvadipine is 2-cyano-5-isopropoxycarbonyl-3-methoxycarbonyl-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,338,322, Japanese patent publication No. Sho 52-5777 and the like.
-
Nisoldipine is 2,6-dimethyl-3-isobutoxycarbonyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 4,154,839, Japanese patent publication No. Sho 52-59161 and the like.
-
Nitrendipine is 3-ethoxycarbonyl-2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine disclosed in USP 3,799,934, Japanese patent publication (after examination) No. Sho 55-27054 and the like.
-
Pranidipine is 2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-3-(3-phenyl-2-propen-1 -yloxycarbonyl)-1,4-dihydropyridine disclosed in USP 5,034,395, Japanese patent publication No. Sho 60-120861 and the like.
Process for synthesis of chiral 3-substituted tetrahydroquinoline derivatives……..WO 2013140419…CSIR INDIA PATENT

sumanirole
179386-43-7
179386-44-8 (maleate)

Sumanirole maleate, U-95666 (free base), U-95666E, PNU-95666E
| Process for synthesis of chiral 3-substituted tetrahydroquinoline derivatives | |
| Council Of Scientific & Industrial Research | |
| The present invention relates to novel and concise process for the construction of chiral 3-substituted tetrahydroquinoline derivatives based on proline catalyzed asymmetric α-functionalization of aldehyde, followed by in situ reductive cyclization of nitro group under catalytic hydrogenation condition with high optical purities. Further the invention relates to conversion of derived chiral 3-substituted tetrahydroquinoline derivatives into therapeutic agents namely (-)-sumanirole (96% ee) and 1-[(S)-3-(dimethylamino)-3,4-dihydro-6,7-dimethoxy-quinolin-1(2H)-yl]propanone[(S)-903] (92% ee). | |
| Process,sumanirole | |
| Indications | Restless legs syndrome; Parkinsons disease |
| Target-based Actions | Dopamine D2 receptor agonist |
| Other Actions | Anxiolytic; Antiparkinsonian |
 |
|
| Inventors | Boopathi, Senthil, Kumar; Arumugam, Sudalai; Rawat, Varun |
| IPC Codes | C07D 215/20; C07D 471/06; C07D 215/38 |
| DRUG | sumanirole |
| Publication Date | 26-Sep-2013 WO-2013140419-A1 |
Sumanirole (PNU-95,666) is a highly selective D2 receptor full agonist, the first of its kind to be discovered. It was developed for the treatment of Parkinson’s disease andrestless leg syndrome. While it has never been approved for medical use it is a highly valuable tool compound for basic research to identify neurobiological mechanisms that are based on a dopamine D2-linked (vs. D1, D3, D4, and D5-linked) mechanism of action
sumanirole
OTHER INFO

D-Phenylalanine (I) was protected as the methyl carbamate (II) by acylation with methyl chloroformate under Schotten-Baumann conditions. The N-methoxy amide (III) was then prepared by coupling of (II) with O-methyl hydroxylamine in the presence of EDC. Cyclization of (III) to the N-methoxy quinolinone (IV) was accomplished by treatment with bis(trifluoroacetoxy)iodobenzene in the presence of trifluoroacetic acid. Simultaneous reduction of the N-methoxy lactam and carbamate functions of (IV) by means of borane-methyl sulfide complex provided diamine (V). The aliphatic amino group of (V) was then selectively protected as the benzyl carbamate (VI) by using N-(benzyloxycarbonyloxy)succinimide at -40 C. Reaction of (VI) with phosgene, followed by treatment of the intermediate carbamoyl chloride with O-methyl hydroxylamine gave rise to the N-methoxy urea derivative (VII). This was cyclized with bis(trifluoroacetoxy)iodobenzene to the imidazoquinolinone (VIII). The N-methoxy and N-benzyloxycarbonyl groups of (VIII) were then removed by hydrogenolysis in the presence of Pearlman’s catalyst, and the title compound was finally converted to the corresponding maleate salt.
JOC 1997,62,(19):6582
Rheumatoid arthritis and osteoarthritis
Roche Gets Breakthrough Status for Lung Cancer Drug
ALECTINIB
http://www.who.int/medicines/publications/druginformation/issues/PL_108.pdf
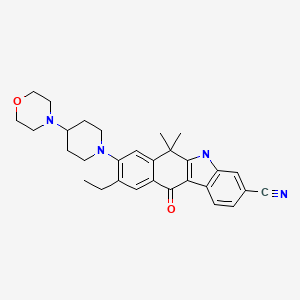
9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-
6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile
tyrosine kinase inhibitor, antineoplastic
C30H34N4O2, CAS 1256580-46-7
The U.S. Food and Drug Administration (FDA) has granted breakthrough therapy designation for Roche’s alectinib – a promising investigational 2nd generation ALK inhibitor – based on data that will be presented at European Cancer Congress (ECC). Read more…http://www.dddmag.com/news/2013/09/roche-gets-breakthrough-status-lung-cancer-drug?et_cid=3497158&et_rid=523035093&type=cta

BIOSIMILARS
The strategy of enantiomer patents of drugs

The strategy of enantiomer patents of drugs
Drug Discovery Today, Volume 15, Issues 5–6, March 2010, Pages 163-170
http://www.sciencedirect.com/science/article/pii/S1359644610000310
- Organic Chemistry, Institute of Chemistry, The Hebrew University of Jerusalem, Philadelphia Building 201/205, Edmond J. Safra Campus, Jerusalem 91904, Israel
Enantiomer patents (ENPTs), constituents of chiral switches, claim single enantiomers of chiral drugs previously claimed as racemates. In this article, the strategy of ENPTs and recent court decisions and trends in case law worldwide are highlighted. ENPTs are challenged frequently (e.g. anticipation, obviousness, double patenting and insufficient disclosure), even though the novelty of enantiomers is not destroyed by the description of racemates. For establishing inventiveness (nonobviousness), the description in ENPTs should include superior pharmacological and/or pharmaceutical properties of enantiomer vis-á-visracemate, above the expected 2:1 ratio. ENPTs were ‘obvious-to-try’ (unless taught away) since the mid-1980s. General concern about evergreening by ENPTs is not justified. ENPTs should be evaluated on a case-by-case basis. ENPT litigations are especially susceptible to settlements.