- WhaTech Channel: Reports Industrial
- Published: Wednesday, 18 June 2014 20:34
- Submitted by Market Research WhaTech Premium
- News from: RnR Market Research
Hepatitis C market by 2018 is expected to grow more than 3 times from its current market size of 2012. Hepatitis C virus (HCV) infection is a complex public health problem, characterized by a high prevalence of chronic infection, an increasing burden of HCV-associated disease, low rates of testing, treatment and the prospect of increasing incidence associated with the epidemic of injection drug use.
Hepatitis C Market Overview
Hepatitis C is a leading cause of chronic liver disease, end-stage cirrhosis and liver cancer. Because of the slow progression and asymptomatic character of the infection, many people are unaware of having it. As a consequence, the infection is often diagnosed at a late stage when treatment options are limited. As no effective vaccine against the Hepatitis C virus (HCV) has been discovered so far the market is driven by therapeutics. The increase in the prevalence of the disease and the availability of new first-in-class therapies with better safety and efficacy profiles are expected to drive the growth of the HCV market. The growth in HCV drugs market is primarily attributed to high unmet need in the market which is expected to be fulfilled by strong pipeline candidates. Low levels of awareness and knowledge about HCV have been identified as a formidable challenge to prevention and care.
Complete report is available @ http://www.rnrmarketresearch.com/hepatitis-c-market-forecast-hcv-drugs-clinical-trials-hepatitis-c-pipeline-drugs-sales-forecast-worldwide-market-report.html .
Renub Research study titled “Hepatitis C Market & Forecast, HCV Drugs Clinical Trials, Hepatitis C Pipeline Drugs Sales & Forecast – Worldwide” provides a comprehensive assessment of the fast-evolving, high-growth of Hepatitis sector. This 164 page report with 25 Figures and 2 Tables studies the Hepatitis C Drug Market Landscape. This report contains 7 chapters.
- Hepatitis C Drugs Market & Forecast (Chapter 2)
- Hepatitis C Approved Drugs Sales & Forecast (Chapter 3)
- Hepatitis C Deals & Acquisitions (Chapter 4)
- Hepatitis C – Pipeline Drugs Clinical Trials (Drugs in Phase III) (Chapter 5)
- Hepatitis C – Pipeline Drugs Clinical Trials (Drugs in Phase II) (Chapter 6)
- Hepatitis C – Pipeline Drugs Sales Forecast (Chapter 7)
Hepatitis C – Approved Drugs sales & Forecast Analysis (To 2016) (Chapter No. 3)
- Pegasys
- Pegintron
- Incivek
- Victrelis
Hepatitis C – Pipeline Drugs Clinical Trials (Drugs in Phase III) (Chapter No. 5)
- Simeprevir (TMC 435) (Company: Janssen Pharmaceutical)
- Faldaprevir (BI 201335) (Company: Boerhinger Ingelheim)
- Asunaprevir (BMS-650032) (Company: Bristol-Myers Squibb)
- PEG-Interferon Lambda (Company: Bristol-Myers Squibb)
- Sofosbuvir (PSI-7977 or GS-7977) (Company: Gilead Sciences)
- Daclatasvir (BMS-790052) (Company: Bristol-Myers Squibb)
- BI-207127 (Company: Boerhinger Ingelheim)
- ABT-450/r (Ritonavir) (Company: Abbott Laboratories)
- ABT-267 (Company: Abbott Laboratories)
- ABT-072/333 (Company: Abbott Laboratories)
- Alisporivir (Company: Novartis)
Purchase a copy of this report @ http://www.rnrmarketresearch.com/contacts/purchase?rname=186104 .
Hepatitis C – Pipeline Drugs Clinical Trials (Drugs in Phase II) (Chapter No. 6)
- Mericitabine (RG-7128) (Company: Roche)
- Danoprevir/r (Ritonavir) (RG7227) (Company: Roche)
- GS-9256 (Company: Gilead Sciences)
- GS-9451 (Company: Gilead Sciences)
- MK-5172 (Company: Merck)
- Sovaprevir (ACH-1625) (Company: Achillion)
- IDX-320 (Company: Idenix)
- MK-8742 (Company: Merck)
- ACH-3102 (Company: Achillion Pharmaceuticals, Inc)
- IDX-719 (Company: Idenix)
- PPI-668 (Company: Presidio Pharmaceuticals)
- Setrobuvir (ANA-598) (Company: Roche)
- VX-222 (Company: Vertex Pharmaceuticals)
- GS-9669 (Company: Gilead Sciences)
- GS-9190 (Tegobuvir) (Company: Gilead Sciences)
- BMS-791325 (Company: Bristol-Myers Squibb)
Hepatitis C – Pipeline Drugs Sales Forecast (Chapter 7)
- Simeprevir (TMC 435)
- Faldaprevir (BI 201335) (Boerhinger Ingelheim)
- Asunaprevir (BMS-650032)
- Sofosbuvir (PSI-7977 or GS-7977)
- Daclatasvir (BMS-790052)
- ABT-450/r (Ritonavir)
- ABT-072/333
- Alisporivir
- Mericitabine (RG-7128)
- Danoprevir (RG7227)
- GS-9256
- Setrobuvir (ANA-598)
- VX-222
- GS-9190 (Tegobuvir)
- BMS-791325
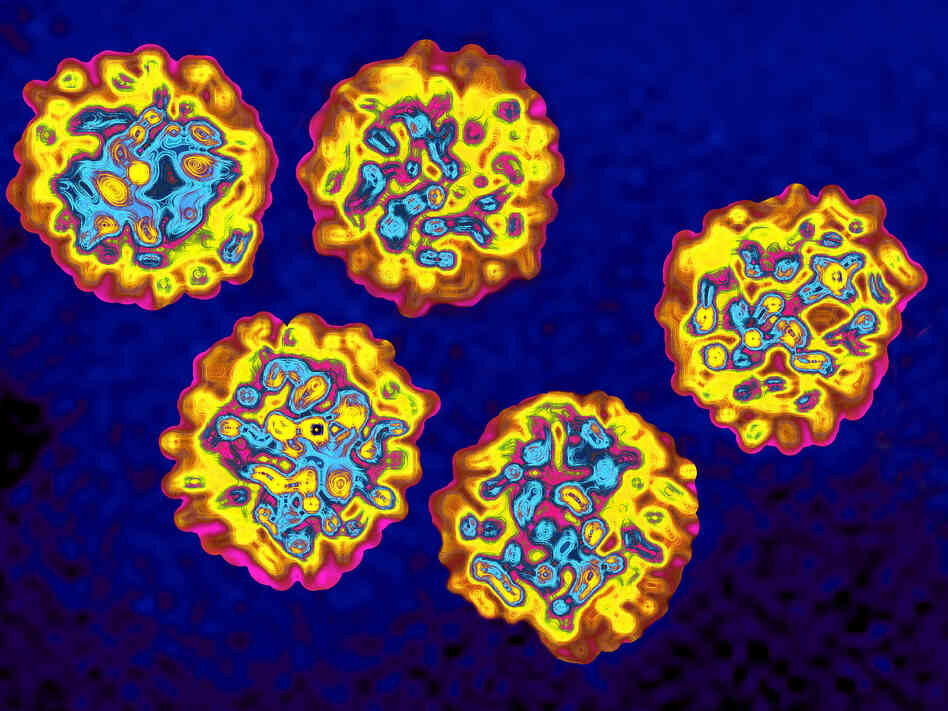
Data Sources
This report is built using data and information sourced from proprietary databases, primary and secondary research and in-house analysis by Renub Research team of industry experts.
Primary sources include industry surveys and telephone interviews with industry experts.
Secondary sources information and data has been collected from various printable and non-printable sources like search engines, News websites, Government Websites, Trade Journals, White papers, Government Agencies, Magazines, Newspapers, Trade associations, Books, Industry Portals, Industry Associations and access to more than 500 paid databases.
For more information:



























 Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -
Cannabidiol
Seven Expanded Access INDs granted by FDA to U.S.
physicians to treat with Epidiolex 125 children suffering
from intractable epilepsy syndromes -