
Cystic Fibrosis Letdown Explained Drug Combo: Cell studies suggest way to boost clinical outcomes


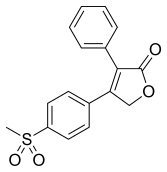
ROFECOXIB
MK-966, MK-0966, Vioxx
162011-90-7
Rofecoxib /ˌrɒfɨˈkɒksɪb/ is a nonsteroidal anti-inflammatory drug (NSAID) that has now been withdrawn over safety concerns. It was marketed by Merck & Co. to treat osteoarthritis, acute pain conditions, and dysmenorrhoea. Rofecoxib was approved by the Food and Drug Administration (FDA) on May 20, 1999, and was marketed under the brand names Vioxx, Ceoxx, and Ceeoxx.
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 4-(4-methylsulfonylphenyl)-3-phenyl-5H-furan-2-one | |
| Clinical data | |
| Pregnancy cat. | C (AU) |
| Legal status | Prescription Only (S4) (AU)withdrawn |
| Routes | oral |
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | 87% |
| Metabolism | hepatic |
| Half-life | 17 hours |
| Excretion | biliary/renal |
| Identifiers | |
| CAS number | 162011-90-7 |
| ATC code | M01AH02 |
| PubChem | CID 5090 |
| DrugBank | DB00533 |
| ChemSpider | 4911 |
| UNII | 0QTW8Z7MCR |
| Chemical data | |
| Formula | C17H14O4S |
| Mol. mass | 314.357 g/mol |
Rofecoxib gained widespread acceptance among physicians treating patients with arthritis and other conditions causing chronic or acute pain. Worldwide, over 80 million people were prescribed rofecoxib at some time.[1]
On September 30, 2004, Merck withdrew rofecoxib from the market because of concerns about increased risk of heart attack and stroke associated with long-term, high-dosage use. Merck withdrew the drug after disclosures that it withheld information about rofecoxib’s risks from doctors and patients for over five years, resulting in between 88,000 and 140,000 cases of serious heart disease.[2] Rofecoxib was one of the most widely used drugs ever to be withdrawn from the market. In the year before withdrawal, Merck had sales revenue of US$2.5 billion from Vioxx.[3] Merck reserved $970 million to pay for its Vioxx-related legal expenses through 2007, and have set aside $4.85bn for legal claims from US citizens.
Rofecoxib was available on prescription in both tablet-form and as an oral suspension. It was available by injection for hospital use.
Cyclooxygenase (COX) has two well-studied isoforms, called COX-1 and COX-2. COX-1 mediates the synthesis of prostaglandinsresponsible for protection of the stomach lining, while COX-2 mediates the synthesis of prostaglandins responsible for pain and inflammation. By creating “selective” NSAIDs that inhibit COX-2, but not COX-1, the same pain relief as traditional NSAIDs is offered, but with greatly reduced risk of fatal or debilitating peptic ulcers. Rofecoxib is a selective COX-2 inhibitor, or “coxib”.
Others include Merck’s etoricoxib (Arcoxia), Pfizer’s celecoxib (Celebrex) and valdecoxib (Bextra). Interestingly, at the time of its withdrawal, rofecoxib was the only coxib with clinical evidence of its superior gastrointestinal adverse effect profile over conventional NSAIDs. This was largely based on the VIGOR (Vioxx GI Outcomes Research) study, which compared the efficacy and adverse effect profiles of rofecoxib and naproxen.[4]
The therapeutic recommended dosages were 12.5, 25, and 50 mg with an approximate bioavailability of 93%.[5][6][7] Rofecoxib crossed the placenta and blood–brain barrier,[5][6][8]and took 1–3 hours to reach peak plasma concentration with an effective half-life (based on steady-state levels) of approximately 17 hours.[5][7][9] The metabolic products are cis-dihydro and trans-dihydro derivatives of rofecoxib[5][9] which are primarily excreted through urine.
On March 11, 2009, Scott S. Reuben, former chief of acute pain at Baystate Medical Center, Springfield, Mass., revealed that data for 21 studies he had authored for the efficacy of the drug (along with others such as celecoxib) had been fabricated in order to augment the analgesic effects of the drugs. There is no evidence that Reuben colluded with Merck in falsifying his data. Reuben was also a former paid spokesperson for the drug company Pfizer (which owns the intellectual property rights for marketing celecoxib in the United States). The retracted studies were not submitted to either the FDA or the European Union’s regulatory agencies prior to the drug’s approval. Drug manufacturer Merckhad no comment on the disclosure.[10]
Aside from the reduced incidence of gastric ulceration, rofecoxib exhibits a similar adverse effect profile to other NSAIDs.
Prostaglandin is a large family of lipids. Prostaglandin I2/PGI2/prostacyclin is just one member of it. Prostaglandins other than PGI2 (such as PGE2) also play important roles in vascular tone regulation. Prostacyclin/thromboxane are produced by both COX-1 and COX-2, and rofecoxib suppresses just COX-2 enzyme, so there is no reason to believe that prostacyclin levels are significantly reduced by the drug. And there is no reason to believe that only the balance between quantities of prostacyclin and thromboxane is the determinant factor for vascular tone.[11] Indeed Merck has stated that there was no effect on prostacyclin production in blood vessels in animal testing.[12] Other researchers have speculated that the cardiotoxicity may be associated with maleic anhydride metabolites formed when rofecoxib becomes ionized under physiological conditions. (Reddy & Corey, 2005)
The VIGOR (Vioxx GI Outcomes Research) study, conducted by Bombardier, et al., which compared the efficacy and adverse effect profiles of rofecoxib and naproxen, had indicated a significant 4-fold increased risk of acute myocardial infarction (heart attack) in rofecoxib patients when compared with naproxen patients (0.4% vs 0.1%, RR 0.25) over the 12 month span of the study. The elevated risk began during the second month on rofecoxib. There was no significant difference in the mortality from cardiovascular events between the two groups, nor was there any significant difference in the rate of myocardial infarction between the rofecoxib and naproxen treatment groups in patients without high cardiovascular risk. The difference in overall risk was by the patients at higher risk of heart attack, i.e. those meeting the criteria for low-dose aspirin prophylaxis of secondary cardiovascular events (previous myocardial infarction, angina, cerebrovascular accident, transient ischemic attack, or coronary artery bypass).
Merck’s scientists interpreted the finding as a protective effect of naproxen, telling the FDA that the difference in heart attacks “is primarily due to” this protective effect (Targum, 2001). Some commentators have noted that naproxen would have to be three times as effective as aspirin to account for all of the difference (Michaels 2005), and some outside scientists warned Merck that this claim was implausible before VIGOR was published.[13] No evidence has since emerged for such a large cardioprotective effect of naproxen, although a number of studies have found protective effects similar in size to those of aspirin.[14][15] Though Dr. Topol’s 2004 paper criticized Merck’s naproxen hypothesis, he himself co-authored a 2001 JAMA article stating “because of the evidence for an antiplatelet effect of naproxen, it is difficult to assess whether the difference in cardiovascular event rates in VIGOR was due to a benefit from naproxen or to a prothrombotic effect from rofecoxib.” (Mukherjee, Nissen and Topol, 2001.)
The results of the VIGOR study were submitted to the United States Food and Drug Administration (FDA) in February 2001. In September 2001, the FDA sent a warning letter to the CEO of Merck, stating, “Your promotional campaign discounts the fact that in the VIGOR study, patients on Vioxx were observed to have a four to five fold increase in myocardial infarctions (MIs) compared to patients on the comparator non-steroidal anti-inflammatory drug (NSAID), Naprosyn (naproxen).”[16] This led to the introduction, in April 2002, of warnings on Vioxx labeling concerning the increased risk of cardiovascular events (heart attack and stroke).
Months after the preliminary version of VIGOR was published in the New England Journal of Medicine, the journal editors learned that certain data reported to the FDA were not included in the NEJM article. Several years later, when they were shown a Merck memo during the depositions for the first federal Vioxx trial, they realized that these data had been available to the authors months before publication. The editors wrote an editorial accusing the authors of deliberately withholding the data.[17] They released the editorial to the media on December 8, 2005, before giving the authors a chance to respond. NEJM editor Gregory Curfman explained that the quick release was due to the imminent presentation of his deposition testimony, which he feared would be misinterpreted in the media. He had earlier denied any relationship between the timing of the editorial and the trial. Although his testimony was not actually used in the December trial, Curfman had testified well before the publication of the editorial.[18]
The editors charged that “more than four months before the article was published, at least two of its authors were aware of critical data on an array of adverse cardiovascular events that were not included in the VIGOR article.” These additional data included three additional heart attacks, and raised the relative risk of Vioxx from 4.25-fold to 5-fold. All the additional heart attacks occurred in the group at low risk of heart attack (the “aspirin not indicated” group) and the editors noted that the omission “resulted in the misleading conclusion that there was a difference in the risk of myocardial infarction between the aspirin indicated and aspirin not indicated groups.” The relative risk for myocardial infarctions among the aspirin not indicated patients increased from 2.25 to 3 (although it remained statitistically insignificant). The editors also noted a statistically significant (2-fold) increase in risk for serious thromboembolic events for this group, an outcome that Merck had not reported in the NEJM, though it had disclosed that information publicly in March 2000, eight months before publication.[19]
The authors of the study, including the non-Merck authors, responded by claiming that the three additional heart attacks had occurred after the prespecified cutoff date for data collection and thus were appropriately not included. (Utilizing the prespecified cutoff date also meant that an additional stroke in the naproxen population was not reported.) Furthermore, they said that the additional data did not qualitatively change any of the conclusions of the study, and the results of the full analyses were disclosed to the FDA and reflected on the Vioxx warning label. They further noted that all of the data in the “omitted” table were printed in the text of the article. The authors stood by the original article.[20]
NEJM stood by its editorial, noting that the cutoff date was never mentioned in the article, nor did the authors report that the cutoff for cardiovascular adverse events was before that for gastrointestinal adverse events. The different cutoffs increased the reported benefits of Vioxx (reduced stomach problems) relative to the risks (increased heart attacks).[19]
Some scientists have accused the NEJM editorial board of making unfounded accusations.[21][22] Others have applauded the editorial. Renowned research cardiologist Eric Topol,[23] a prominent Merck critic, accused Merck of “manipulation of data” and said “I think now the scientific misconduct trial is really fully backed up”.[24] Phil Fontanarosa, executive editor of the prestigious Journal of the American Medical Association, welcomed the editorial, saying “this is another in the long list of recent examples that have generated real concerns about trust and confidence in industry-sponsored studies”.[25]
On May 15, 2006, the Wall Street Journal reported that a late night email, written by an outside public relations specialist and sent to Journal staffers hours before the Expression of Concern was released, predicted that “the rebuke would divert attention to Merck and induce the media to ignore the New England Journal of Medicine‘s own role in aiding Vioxx sales.”[26]
“Internal emails show the New England Journal’s expression of concern was timed to divert attention from a deposition in which Executive Editor Gregory Curfman made potentially damaging admissions about the journal’s handling of the Vioxx study. In the deposition, part of the Vioxx litigation, Dr. Curfman acknowledged that lax editing might have helped the authors make misleading claims in the article.” The Journal stated that NEJM‘s “ambiguous” language misled reporters into incorrectly believing that Merck had deleted data regarding the three additional heart attacks, rather than a blank table that contained no statistical information; “the New England Journal says it didn’t attempt to have these mistakes corrected.”[26]
In 2000 and 2001, Merck conducted several studies of rofecoxib aimed at determining if the drug slowed the onset of Alzheimer’s disease. Merck has placed great emphasis on these studies on the grounds that they are relatively large (almost 3000 patients) and compared rofecoxib to a placebo rather than to another pain reliever. These studies found an elevated death rate among rofecoxib patients, although the deaths were not generally heart-related. However, they did not find any elevated cardiovascular risk due to rofecoxib.[27] Before 2004, Merck cited these studies as providing evidence, contrary to VIGOR, of rofecoxib’s safety.
In 2001, Merck commenced the APPROVe (Adenomatous Polyp PRevention On Vioxx) study, a three-year trial with the primary aim of evaluating the efficacy of rofecoxib for theprophylaxis of colorectal polyps. Celecoxib had already been approved for this indication, and it was hoped to add this to the indications for rofecoxib as well. An additional aim of the study was to further evaluate the cardiovascular safety of rofecoxib.
The APPROVe study was terminated early when the preliminary data from the study showed an increased relative risk of adverse thrombotic cardiovascular events (includingheart attack and stroke), beginning after 18 months of rofecoxib therapy. In patients taking rofecoxib, versus placebo, the relative risk of these events was 1.92 (rofecoxib 1.50 events vs placebo 0.78 events per 100 patient years). The results from the first 18 months of the APPROVe study did not show an increased relative risk of adverse cardiovascular events. Moreover, overall and cardiovascular mortality rates were similar between the rofecoxib and placebo populations.[28]
In summary, the APPROVe study suggested that long-term use of rofecoxib resulted in nearly twice the risk of suffering a heart attack or stroke compared to patients receiving a placebo.
Pre-approval Phase III clinical trials, like the APPROVe study, showed no increased relative risk of adverse cardiovascular events for the first eighteen months of rofecoxib usage (Merck, 2004). Others have pointed out that “study 090,” a pre-approval trial, showed a 3-fold increase in cardiovascular events compared to placebo, a 7-fold increase compared to nabumetone (another [NSAID]), and an 8-fold increase in heart attacks and strokes combined compared to both control groups.[29][30] Although this was a relatively small study and only the last result was statistically significant, critics have charged that this early finding should have prompted Merck to quickly conduct larger studies of rofecoxib’s cardiovascular safety. Merck notes that it had already begun VIGOR at the time Study 090 was completed. Although VIGOR was primarily designed to demonstrate new uses for rofecoxib, it also collected data on adverse cardiovascular outcomes.
Several very large observational studies have also found elevated risk of heart attack from rofecoxib. For example, a recent retrospective study of 113,000 elderly Canadians suggested a borderline statistically significant increased relative risk of heart attacks of 1.24 from Vioxx usage, with a relative risk of 1.73 for higher-dose Vioxx usage. (Levesque, 2005). Another study, using Kaiser Permanente data, found a 1.47 relative risk for low-dose Vioxx usage and 3.58 for high-dose Vioxx usage compared to current use of celecoxib, though the smaller number was not statistically significant, and relative risk compared to other populations was not statistically significant. (Graham, 2005).
Furthermore, a more recent meta-study of 114 randomized trials with a total of 116,000+ participants, published in JAMA, showed that Vioxx uniquely increased risk of renal (kidney) disease, and heart arrhythmia.[31]
Any increased risk of renal and arrhythmia pathologies associated with the class of COX-2 inhibitors, e.g. celecoxib (Celebrex), valdecoxib (Bextra), parecoxib (Dynastat),lumiracoxib, and etoricoxib is not evident,[31] although smaller studies[32][33] had demonstrated such effects earlier with the use of celecoxib, valdecoxib and parecoxib.
Nevertheless, it is likely that trials of newer drugs in the category will be extended in order to supply additional evidence of cardiovascular safety. Examples are some more specific COX-2 inhibitors, including etoricoxib (Arcoxia) and lumiracoxib (Prexige), which are currently (circa 2005) undergoing Phase III/IV clinical trials.
Besides, regulatory authorities worldwide now require warnings about cardiovascular risk of COX-2 inhibitors still on the market. For example, in 2005, EU regulators required the following changes to the product information and/or packaging of all COX-2 inhibitors:[34]
Since the withdrawal of Vioxx it has come to light that there may be negative cardiovascular effects with not only other COX-2 inhibitiors, but even the majority of other NSAIDs. It is only with the recent development of drugs like Vioxx that drug companies have carried out the kind of well executed trials that could establish such effects and these sort of trials have never been carried out in older “trusted” NSAIDs such as ibuprofen, diclofenac and others. The possible exceptions may be aspirin and naproxen due to their anti-platelet aggregation properties.
Due to the findings of its own APPROVe study, Merck publicly announced its voluntary withdrawal of the drug from the market worldwide on September 30, 2004.[35]
In addition to its own studies, on September 23, 2004 Merck apparently received information about new research by the FDA that supported previous findings of increased risk of heart attack among rofecoxib users (Grassley, 2004). FDA analysts estimated that Vioxx caused between 88,000 and 139,000 heart attacks, 30 to 40 percent of which were probably fatal, in the five years the drug was on the market.[36]
On November 5, the medical journal The Lancet published a meta-analysis of the available studies on the safety of rofecoxib (Jüni et al., 2004). The authors concluded that, owing to the known cardiovascular risk, rofecoxib should have been withdrawn several years earlier. The Lancet published an editorial which condemned both Merck and the FDA for the continued availability of rofecoxib from 2000 until the recall. Merck responded by issuing a rebuttal of the Jüni et al. meta-analysis that noted that Jüni omitted several studies that showed no increased cardiovascular risk. (Merck & Co., 2004).
In 2005, advisory panels in both the U.S. and Canada encouraged the return of rofecoxib to the market, stating that rofecoxib’s benefits outweighed the risks for some patients. The FDA advisory panel voted 17-15 to allow the drug to return to the market despite being found to increase heart risk. The vote in Canada was 12-1, and the Canadian panel noted that the cardiovascular risks from rofecoxib seemed to be no worse than those from ibuprofen—though the panel recommended that further study was needed for all NSAIDs to fully understand their risk profiles. Notwithstanding these recommendations, Merck has not returned rofecoxib to the market.[37]
In 2005, Merck retained Debevoise & Plimpton LLP to investigate Vioxx study results and communications conducted by Merck. Through the report, it was found that Merck’s senior management acted in good faith, and that the confusion over the clinical safety of Vioxx was due to the sales team’s overzealous behavior. The report that was filed gave a timeline of the events surrounding Vioxx and showed that Merck intended to operate honestly throughout the process. Any mistakes that were made regarding the mishandling of clinical trial results and withholding of information was the result of oversight, not malicious behavior. The Martin Report did conclude that the Merck’s marketing team exaggerated the safety of Vioxx and replaced truthful information with sales tactics.[citation needed] The report was published in February 2006, and Merck was satisfied with the findings of the report and promised to consider the recommendations contained in the Martin Report. Advisers to the US Food and Drug Administration (FDA) have voted, by a narrow margin, that it should not ban Vioxx — the painkiller withdrawn by drug-maker Merck.
They also said that Pfizer’s Celebrex and Bextra, two other members of the family of painkillers known as COX-2 inhibitors, should remain available, despite the fact that they too boost patients’ risk of heart attack and stroke. url = http://www.nature.com/drugdisc/news/articles/433790b.html The recommendations of the arthritis and drug safety advisory panel offer some measure of relief to the pharmaceutical industry, which has faced a barrage of criticism for its promotion of the painkillers. But the advice of the panel, which met near Washington DC over 16–18 February, comes with several strings attached.
For example, most panel members said that manufacturers should be required to add a prominent warning about the drugs’ risks to their labels; to stop direct-to-consumer advertising of the drugs; and to include detailed, written risk information with each prescription. The panel also unanimously stated that all three painkillers “significantly increase the risk of cardiovascular events”.
The panel voted 17 to 15 against banning Vioxx (rofecoxib) entirely; the vote on Bextra (valdecoxib) was 17 to 13 with 2 abstentions; Celebrex (celecoxib) was endorsed 31 to 1. Shares of Merck, based in Whitehouse Station, New Jersey, and New York-based Pfizer closed up 13% and 7% respectively on 18 February, 2013, the day of the votes.
The FDA is expected to act on the recommendations within weeks. Although the agency usually follows the recommendations of its outside advisers, it is not bound to do so. A top official said that, in light of the closeness of some of the votes, the agency will examine the panel members’ comments in detail before deciding what to do.
An official from Merck said during the meeting that it would consider reintroducing Vioxx, which it withdrew in September 2004. On April 7, 2005, Pfizer withdrew Bextra from the U.S. market on recommendation by the FDA. Pfizer’s other painkiller, Celebrex, is still on the market.
As of March 2006, there had been over 10,000 cases and 190 class actions filed against Merck[citation needed] over adverse cardiovascular events associated with rofecoxib and the adequacy of Merck’s warnings. The first wrongful death trial, Rogers v. Merck, was scheduled in Alabama in the spring of 2005, but was postponed after Merck argued that the plaintiff had falsified evidence of rofecoxib use.[1]
On August 19, 2005, a jury in Texas voted 10-2 to hold Merck liable for the death of Robert Ernst, a 59-year-old man who allegedly died of a rofecoxib-induced heart attack. The plaintiffs’ lead attorney was Mark Lanier. Merck argued that the death was due to cardiac arrhythmia, which had not been shown to be associated with rofecoxib use. The jury awarded Carol Ernst, widow of Robert Ernst, $253.4 million in damages. This award will almost certainly be capped at no more than US$26.1 million because of punitive damages limits under Texas law.[2] As of March 2006, the plaintiff had yet to ask the court to enter a judgment on the verdict; Merck has stated that it will appeal.
On November 3, 2005, Merck won the second case Humeston v. Merck, a personal injury case, in Atlantic City, New Jersey. The plaintiff experienced a mild myocardial infarction and claimed that rofecoxib was responsible, after having taken it for two months. Merck argued that there was no evidence that rofecoxib was the cause of Humeston’s injury and that there is no scientific evidence linking rofecoxib to cardiac events with short durations of use. The jury ruled that Merck had adequately warned doctors and patients of the drug’s risk.[3]
The first federal trial on rofecoxib, Plunkett v. Merck, began on November 29, 2005 in Houston. The trial ended in a hung jury and a mistrial was declared on December 12, 2005. According to the Wall Street Journal, the jury hung by an eight to one majority, favoring the defense. Upon retrial in February 2006 in New Orleans, where the Vioxx multidistrict litigation (MDL) is based, a jury found Merck not liable, even though the plaintiffs had the NEJM editor testify as to his objections to the VIGOR study.
On January 30, 2006, a New Jersey state court dismissed a case brought by Edgar Lee Boyd, who blamed Vioxx for gastrointestinal bleeding that he experienced after taking the drug. The judge said that Boyd failed to prove the drug caused his stomach pain and internal bleeding.
In January 2006, Garza v. Merck began trial in Rio Grande City, Texas. The plaintiff, a 71-year-old smoker with heart disease, had a fatal heart attack three weeks after finishing a one-week sample of rofecoxib. On April 21, 2006 the jury awarded the plaintiff $7 million compensatory and $25 million punitive. The Texas state court of appeals in San Antonio later rules Garza’s fatal heart attack probably resulted from pre-existing health conditions unrelated to his taking of Vioxx, thus reversing the $32 million jury award.[4]
On April 5, 2006, the jury held Merck liable for the heart attack of 77-year-old John McDarby, and awarded Mr McDarby $4.5 million in compensatory damages based on Merck’s failure to properly warn of Vioxx safety risks. After a hearing on April 11, 2006, the jury also awarded Mr McDarby an additional $9 million in punitive damages. The same jury found Merck not liable for the heart attack of 60-year-old Thomas Cona, a second plaintiff in the trial, but was liable for fraud in the sale of the drug to Cona.
Merck has reserved $970 million to pay for its Vioxx-related legal expenses through 2007, and have set aside $4.85bn for legal claims from US citizens. Patients who claim to have suffered as a result of taking Vioxx in countries outside the US are campaigning for this to be extended.
In March 2010, an Australian class-action lawsuit against Merck ruled that Vioxx doubled the risk of heart attacks, and that Merck had breached the Trade Practices Act by selling a drug which was unfit for sale.[38]
In November 2011, Merck announced a civil settlement with the US Attorney’s Office for the District of Massachusetts, and individually with 43 US states and the District of Columbia, to resolve civil claims relating to Vioxx.[5] Under the terms of the settlement, Merck agreed to pay two-thirds of a previously recorded $950 million reserve charge in exchange for release from civil liability. Litigation with seven additional states remains outstanding. Under separate criminal proceedings, Merck plead guilty to a federal misdemeanor charge relating to the marketing of the drug across state lines, incurring a fine of $321.6 million.[6]
Rofecoxib was shown to improve premenstrual acne vulgaris in a placebo controlled study.[39]

Rofecoxib synthesis.[40]
,,,,,,,,,,,,,,,,,
The oxidation of 4- (methylsulfanyl) acetophenone (X) with monoperoxyphthalic acid (MMPP) in dichloro-methane / methanol gives the corresponding sulfone (XI), which is brominated with Br2 / AlCl3 in chloroform, yielding the expected phenacyl bromide ( XII). Finally, this compound is cyclocondensed with phenylacetic acid (I) by means of 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) and triethylamine in acetonitrile. 5) Reaction of [4- (methylsulfonyl ) phenyl] phenylacetyl-ene (XIII) with CO catalyzed by Rh4 (CO) 12 in THF at 100 C in a stainless steel autoclave at 100 Atm pressure, followed by a chromatographic separation in a silicagel column to eliminate the undesired regioisomer.

……………….
The synthesis of rofecoxib can be performed by several different ways: 1) The condensation of phenylacetic acid (I) with ethyl bromoacetate (II) by means of triethylamine in THF yields 2- (phenylacetoxy) acetic acid ethyl ester (III), which is cyclized to the hydroxyfuranone (IV) by means of potassium tert-butoxide in tert-butanol. The reaction of (IV) with triflic anhydride and diisopropylethylamine in dichloro-methane affords the corresponding triflate (V), which by reaction with LiBr in hot acetone yields the bromofuranone (VI) The condensation of (VI) with 4- (methylsulfanyl) phenylboronic acid (VII) by means of Na2CO3 and Pd (Ph3P) 4 in hot toluene gives 4- [4- (methylsulfanyl) -phenyl]. – 3-phenylfuran-2 (5H) -one (VIII), which is finally oxidized with 2KHSO5.KHSO4.K2SO4 (oxone). 2) The intermediate (VIII) can also be obtained by condensation of triflate (V) with boronic acid ( VII) by means of Na2CO3 and Pd (Ph3P) 4 in hot toluene. 3) The intermediate (VIII) can also be synthesized by the reaction of triflate (V) with tetramethylammonium chloride, giving the chlorofuranone (IX), which is then condensed with boronic acid (VII) as before.

|title= (help)[dead link]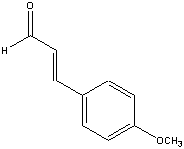
4-Methoxycinnamaldehyde
C10H10O2
NMR Solvent: CDCl3
Note(s):
In the 13C NMR Spectrum, there are two nearly overlapping signals near 127 ppm which are indistinguishable at the initial magnification
MASS
162.1852 MW
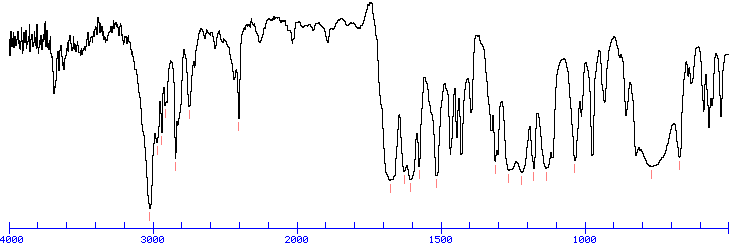
IR SPECTRUM
1H NMR
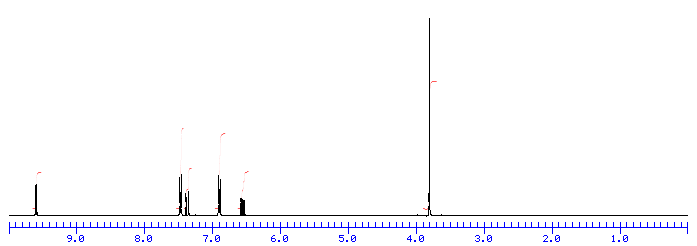
Assign. Shift(ppm)
A 9.651
B 7.523
C 7.422
D 6.947
E 6.610
F 3.857
J(A,E)=8.0HZ
J(C,E)=15.7HZ
DEPT
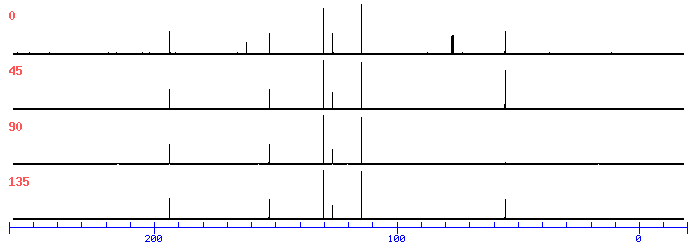
13 C NMR
193.6 C=O
162.2
152.7
130.3
126.7
126.4
114.5
77.4
77.1
76.8
55.4 O-CH3
ppm Int. Assign.
193.41 257 1
162.33 119 2
152.42 259 3
130.34 810 4
126.98 139 5
126.71 464 6
114.68 1000 7
55.47 414 8
|
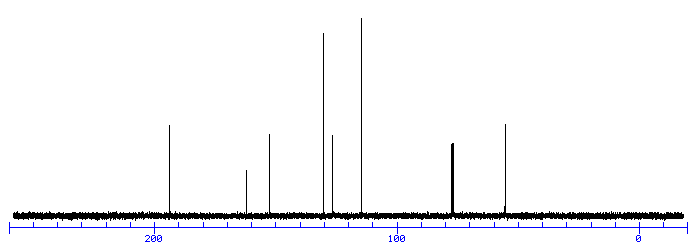
C9H10O2
MW 150
Calculate the degree of unsaturation: the answer is 5. If the degree of unsaturation is 4 or greater, look for an aromatic ring, which has a degree of unsaturation of 4 (3 double bonds plus 1 ring). In addition to an aromatic ring, the molecule can have a carbon-carbon double bond, a carbonyl, or another ring.
Look for a carbonyl, since the degree of unsaturation indicates that the compound could have a double bond and we know that the molecule has an oxygen. There is a band at 1697, suggesting an alpha, beta unsaturated aldehyde or ketone(1710-1665). To see if the compound might be an aldehyde, look for bands in the region 2830-2695. In the spectrum below, note the two bands in this region, suggesting that the compound is indeed an aldehyde.
The IR can also help determine whether or not the compound is an aromatic (although the NMR is a better diagnostic method for this). Look for the C–H stretch in aromatics from 3100-3000. There are a couple very small bands in this region.
There is one more oxygen in the molecule, it could be an ether or even an ester (if we are incorrect in assuming an aldehyde is indicated). Ether IR bands are difficult to distinguish from any other C-O stretch band – the C-O stretch of alcohols, carboxylic acids, esters, and ethers all show up in the region 1320-1000 (see Ethers).
Consult the section on Aromatics for more information on IR spectroscopy of aromatics.

From the IR, we know that compound is probably an aromatic and an aldehyde. Aldehydes and aromatics are quite distinctive in the NMR: aldehydes show up from 9-10, usually as a small singlet; aromatic protons show up from 6.5-8.5 ppm. Let’s look at the NMR:


The singlet at 9.9 ppm indicates an aldehyde; the 4 protons from 7-8 ppm indicate a di-substituted aromatic ring.
The remaining two peaks represent an ethyl group, -CH2CH3: 2 protons split by 3 protons adjacent to 3 protons split by 2 protons. The -CH2– portion of the ethyl group is shifted downfield further than the benzylic protons this indicates that it is next to an oxygen
Let’s draw an 8-carbon 10-hydrogen molecule incorporating these features: an aldehyde, a di-substituted aromatic ring, and an ethyl group:

The molecule is drawn as the para-substituted aromatic; this is indicated by the symmetry of the peaks in the aromatic region.
The following structure with the hydrogens in different colors shows how the protons correlate with the NMR peaks:


NOTE TWO AROM -H ORTHO TO O ATOM APPEAR AT 6.9 PPM
Example is 4-ethoxybenzaldehyde:

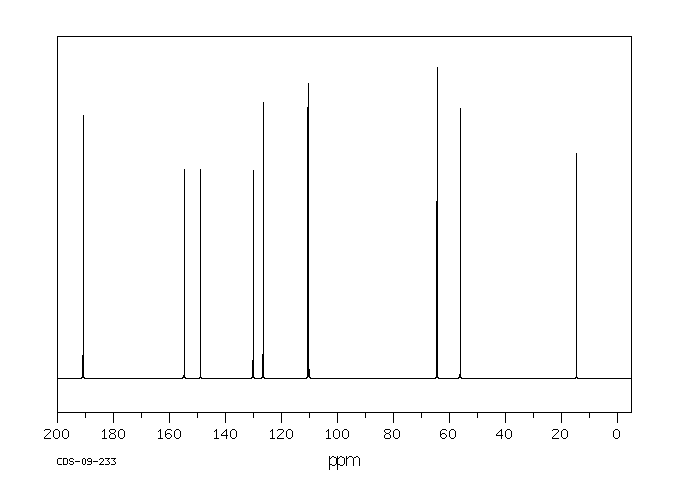
13 C NMR OF 3-Ethoxy-4-methoxybenzaldehyde(1131-52-8)
Alittle complicated than above example
The interpretation is available in form of numbering
13CNMR

Formula: C9H13N, 135.2062

IH NMR

SEE
http://www.sigmaaldrich.com/spectra/fnmr/FNMR006274.PDF
C9H13N
Rule 3, omit the N and one H, gives C9H12
9 – 12/2 + 1 = 4 degrees of unsaturation.
Look for an aromatic ring.
The bands at 3000-2850 indicate C-H alkane stretches. The band at 3028 indicates C-H aromatic stretch; aromatics also show bands in the regions 1600-1585 and 1500-1400 (C-C in-ring stretch), and 900-675 (C-H out-of-plane). The bands in the region 1250-1020 could be due to C-N stretch. The weak, broad banc at about 3500 could be amine N-H stretch or it could be a slight contamination of an impurity (water) in the sample.


 Dedicated to all moms
Dedicated to all moms
C=O group is dad
O atom is mom
Carbonyl is dad and oxygen mom hence c labelled methyl has higher chemical shift and gets a little more attention
SEE BELOW
NMR IS EASY
A chemical has Formula: C5H10O2
C5H10O2
Rule 2, omit O, gives C5H10
5 – 10/2 + 1 = 1 degree of unsaturation.
Look for 1 pi bond or aliphatic ring.
IR
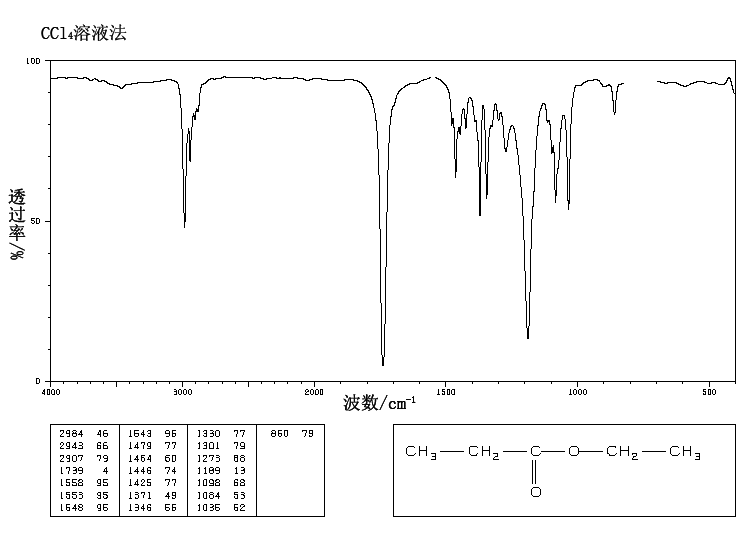

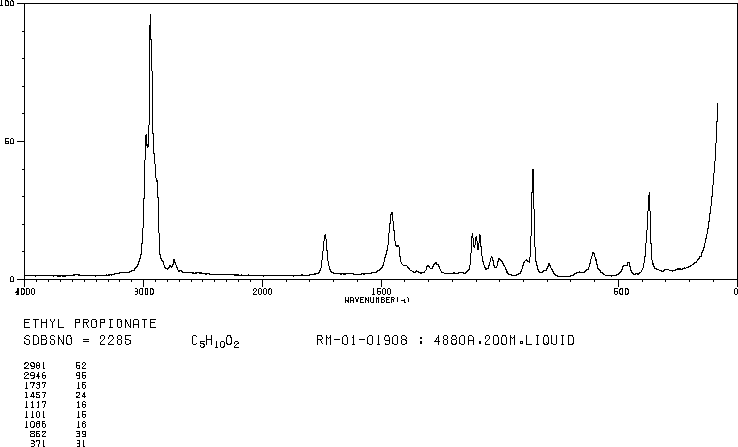
The band at 1740 indicates a carbonyl, probably a saturated aliphatic ester. The bands at 3000-2850 indicate C-H alkane stretches. The bands in the region 1320-1000 could be due to C-O stretch, consistent with an ester.

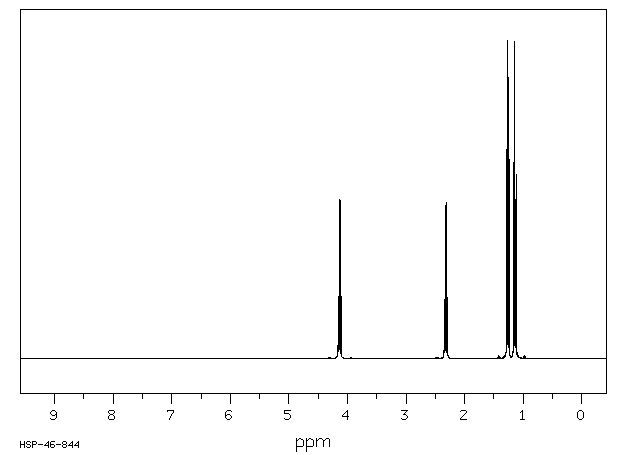
 This is the structure. See if you can assign the peaks on your own.
This is the structure. See if you can assign the peaks on your own. C has a higher chemical shift than D because it’s closer to a more electron-withdrawing functional group.
C has a higher chemical shift than D because it’s closer to a more electron-withdrawing functional group.Carbonyl is dad and oxygen mom, hence c has higher chemical shift and gets a little more attention in proton nmr
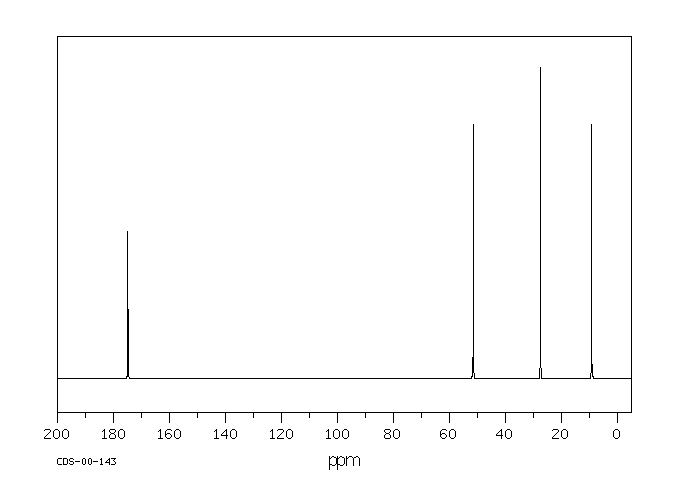
13 C NMR

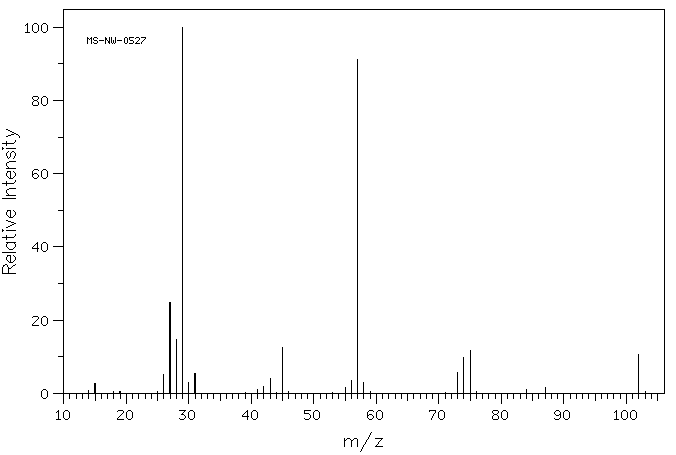
Mass spectrum
RAMAN
WHAT HAPPENS WHEN A CHLORO IS INTRODUCED


WHEN THERE IS ONE METHYL

WHEN THERE ONE CH2 SHORT


WHEN MOM HAS ONE MORE CH2
PROPYL PROPIONATE, try this on your own

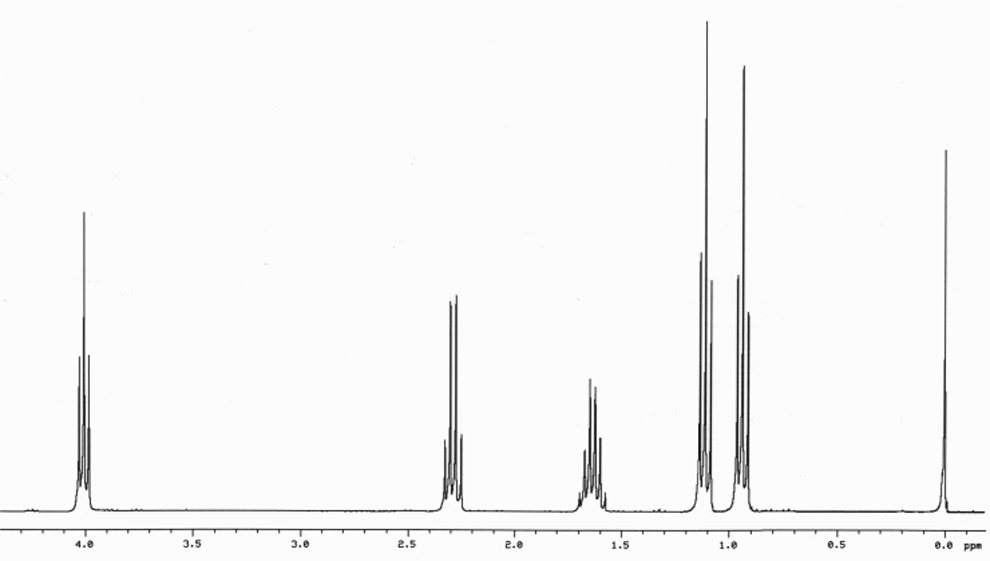
1H NMR

13C NMR

APT

COSY

WILL PASTE INTERPRETATION AFTER ONE WEEK……………….

Dantrolene sodium
1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2,4-imidazolidinedione
FDA Approves Ryanodex for the Treatment of Malignant Hyperthermia
WOODCLIFF LAKE, N.J.(BUSINESS WIRE) July 23, 2014 — Eagle Pharmaceuticals, Inc. (“Eagle” or “the Company”) (Nasdaq:EGRX) today announced that the U. S. Food and Drug Administration (FDA) has approved Ryanodex (dantrolene sodium) for injectable suspension indicated for the treatment of malignant hyperthermia (MH), along with the appropriate supportive measures. MH is an inherited and potentially fatal disorder triggered by certain anesthesia agents in genetically susceptible individuals. FDA had designated Ryanodex as an Orphan Drug in August 2013. Eagle has been informed by the FDA that it will learn over the next four to six weeks if it has been granted the seven year Orphan Drug market exclusivity.
read at
Dantrium Intravenous is a sterile, non-pyrogenic, lyophilized formulation of dantrolene sodium for injection.
Dantrium Intravenous is supplied in 70 mL vials containing 20 mg dantrolene sodium, 3000 mg mannitol,
and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
Dantrium is classified as a direct-acting skeletal muscle relaxant. Chemically, Dantrium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2,4-imidazolidinedione sodium salt. The structural formula for the hydrated salt is:
 |
The hydrated salt contains approximately 15% water (3-1/2 moles) and has a molecular weight of 399. The anhydrous salt (dantrolene) has a molecular weight of 336.
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 1-{[5-(4-nitrophenyl)-2-furyl]methylideneamino} imidazolidine-2,4-dione |
|
| Clinical data | |
| Trade names | Dantrium |
| AHFS/Drugs.com | monograph |
| Pregnancy cat. | C (US) |
| Legal status | ? |
| Routes | Oral, intravenous |
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Metabolism | Liver |
| Excretion | Biliary, renal |
| Identifiers | |
| CAS number | 7261-97-4 |
| ATC code | M03CA01 |
| PubChem | CID 2952 |
| IUPHAR ligand | 4172 |
| DrugBank | DB01219 |
| ChemSpider | 2847 |
| UNII | F64QU97QCR |
| KEGG | D02347 |
| ChEBI | CHEBI:4317 |
| ChEMBL | CHEMBL1201288 |
| Chemical data | |
| Formula | C14H10N4O5 |
| Mol. mass | 314.253 g/mol |
Dantrolene sodium is a muscle relaxant that acts by abolishing excitation-contraction coupling in muscle cells, probably by action on the ryanodine receptor. It is the only specific and effective treatment for malignant hyperthermia, a rare, life-threatening disorder triggered by general anesthesia. It is also used in the management of neuroleptic malignant syndrome, muscle spasticity (e.g. afterstrokes, in paraplegia, cerebral palsy, or patients with multiple sclerosis), 3,4-methylenedioxymethamphetamine (“ecstasy”)intoxication, serotonin syndrome, and 2,4-dinitrophenol poisoning.[1] It is marketed by JHP Pharmaceuticals LLC as Dantrium (in North America) and by Norgine BV as Dantrium, Dantamacrin, or Dantrolen (in Europe).
Dantrolene was first described in the scientific literature in 1967, as one of several hydantoin derivatives proposed as a new class of muscle relaxant.[2] Dantrolene underwent extensive further development, and its action on skeletal muscle was described in detail in 1973.[3]
Dantrolene was widely used in the management of spasticity before its efficacy in treating malignant hyperthermia was discovered by South African anesthesiologist Gaisford Harrison and reported in a landmark 1975 article published in the British Journal of Anaesthesia.[4] Harrison experimentally induced malignant hyperthermia with halothane anesthesia in genetically susceptible pigs, and obtained an 87.5% survival rate, where seven of his eight experiments survived after intravenous administration of dantrolene. The efficacy of dantrolene in humans was later confirmed in a large, multicenter study published in 1982,[5] and confirmed epidemiologically in 1993.[6] Before dantrolene, the only available treatment for malignant hyperthermia was procaine, which was associated with a 60% mortality rate in animal models.[4]
JULY 2014
The US Food and Drug Administration (FDA) has approved an injectable form of dantrolene sodium (Ryanodex, Eagle Pharmaceuticals) for rapid treatment of malignant hyperthermia (MH), along with the appropriate supportive measures, the company announced in a news release today.
MH is a potentially fatal inherited disorder triggered by exposure to certain drugs used for general anesthesia, including the neuromuscular blocking agent succinylcholine.
Ryanodex — which can be administered much more quickly than current formulations of dantrolene — is the first significant enhancement to MH treatment options in more than 3 decades, according to the company.
Ryanodex will be available in single-use vials containing 250 mg of dantrolene sodium in lyophilized powder form. It is formulated for rapid reconstitution and administration in less than 1 minute to patients in MH crisis. “Ryanodex should be administered by continuous rapid intravenous push beginning with a loading dose of 2.5 mg/kg, and continuing until symptoms subside,” the company says.
Ryanodex allows anesthesiologists to deliver a therapeutic dose of dantrolene sodium in a much more expedient manner than currently possible with existing IV formulations of dantrolene sodium, “potentially saving lives and reducing MH-related morbidity,” according to the company.
Other dantrolene sodium formulations require multiple 20-mg vials reconstituted in large volumes of sterile water, a process that can take 15 to 20 minutes to mix reconstitute and administer, the company notes.
MH during surgery is a “life-threatening emergency requiring immediate treatment including the administration of the ‘antidote’ drug dantrolene sodium,” Henry Rosenberg, MD, CPE, a founder and president of the Malignant Hyperthermia Association of the United States, said in the release.
“The ability for healthcare professionals in hospitals and surgery centers to more quickly prepare and administer this new formulation of the antidote dantrolene sodium is expected to bring the crisis under control more rapidly and prevent severe complications from MH,” he said.
The FDA granted Ryanodex orphan drug status in August 2013 and priority review status in March 2014.Ryanodex will be available to order through national and regional drug wholesalers in August with product shipping shortly after. More information is available at http://www.ryanodex.com/.
Oral dantrolene cannot be used by:
If the indication is a medical emergency, such as malignant hyperthermia, the only significant contraindication is hypersensitivity.
If needed in pregnancy, adequate human studies are lacking, therefore the drug should be given in pregnant women only if clearly indicated. It may cause hypotonia in the newborn if given closely before delivery.[1]
Dantrolene should not be given to breastfeeding mothers. If a treatment is necessary, breastfeeding should be terminated.
Central nervous system side effects are quite frequently noted and encompass speech and visual disturbances, mental depression and confusion, hallucinations, headache, insomnia and exacerbation or precipitation of seizures, and increased nervousness. Infrequent cases of respiratory depression or a feeling of suffocation have been observed. Dantrolene often causes sedation severe enough to incapacitate the patient to drive or operate machinery.
Gastrointestinal effects include bad taste, anorexia, nausea, vomiting, abdominal cramps, and diarrhea.
Hepatic side effects may be seen either as asymptomatic elevation of liver enzymes and/or bilirubin or, most severe, as fatal and nonfatal hepatitis. The risk of hepatitis is associated with the duration of treatment and the daily dose. In patients treated for hyperthermia, no liver toxicity has been observed so far.
Pleural effusion with pericarditis (oral treatment only), rare cases of bone marrow damage, diffuse myalgias, backache, dermatologic reactions, transient cardiovascular reactions, and crystalluria have additionally been seen. Muscle weakness may persist for several days following treatment.
Dantrolene gave positive results in animal high dose studies (with and without enzymatic activation) regarding mutagenicity and carcinogenity. No evidence for human mutagenicity and carcinogenity has been found during the long years of clinical experience.
Dantrolene depresses excitation-contraction coupling in skeletal muscle by binding to the ryanodine receptor, and decreasing free intracellular calcium concentration.[1]

Skeletal formula of azumolene. The bromine atom replacing the nitro group found in dantrolene may be seen at left.
Chemically it is a hydantoin derivative, but does not exhibit antiepileptic activity like other hydantoin derivates such as phenytoin.[1]
The poor water solubility of dantrolene leads to certain difficulties in its use.[1][7] A more water-soluble analog of dantrolene, azumolene, is under development for similar indications.[7] Azumolene has a bromine residue instead of the nitro group found in dantrolene, and is 30 times more water-soluble.[1]
…………………………………………….
Bioorganic and medicinal chemistry letters, 2002 , vol. 12, 22 p. 3263 – 3265
http://www.google.co.in/patents/US4543359
…………………………………………………………………………………………………
US4543359
http://www.google.co.in/patents/US4543359
Dantrolene sodium (1-[[5-(p-nitrophenyl) furfurylidene]-amino]hydantoin sodium salt) is described in U.S. Pat. No. 3,415,821. It is used as a skeletal muscle relaxant particularly in controlling the manifestations of clinical spasticity resulting from upper neuron disorders (Physicians’ Desk Reference, 36th Edition, 1982). It is also used in the prevention and treatment of malignant hyperthermia in humans (Friesen et al., Can. Anaesth. Soc. J. 26:319-321, 1979). In connection with the use of dantrolene sodium in hyperthermic crisis it was observed that there was an elimination of the arrhythmias accompanying such crisis [Salata et al., Effects of Dantrolene Sodium on the Electrophysiological Properties of Canine Cardiac Purkinje Fibers, J. Pharmacol. Exp. Ther. 220(1):157-166 (Jan.) 1982] incorporated herein by reference.
………………………………………….
Dantrolene may interact with the following drugs:[8]
| Reference | ||
|---|---|---|
| 1 | * | Dissertation Abstracts International, 42(4), 1337 B, (1981), Malloy, K., PH.D. Thesis, 1981 . |
| 2 | Dissertation Abstracts International, 42(4), 1337-B, (1981), [Malloy, K., PH.D. Thesis, 1981]. | |
| 3 | * | Dissertation Abstracts International, 42(8), 3222 B, (1982), Salata, J., Ph.D. Thesis, 1981 . |
| 4 | Dissertation Abstracts International, 42(8), 3222-B, (1982), [Salata, J., Ph.D. Thesis, 1981]. | |
| 5 | * | Malloy, K., Ph.D. Thesis, Univ. of Rochester, 1981. |
| 6 | * | Salata, J. et al., J. Pharmacol. Exp. Ther., 220(1), 157 166, (1982). |
| 7 | Salata, J. et al., J. Pharmacol. Exp. Ther., 220(1), 157-166, (1982). | |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US4822629 * | 12 Dec 1986 | 18 Apr 1989 | Norwich Eaton Pharmaceuticals, Inc. | Azumolene dosage form |
| US4837163 * | 2 Oct 1987 | 6 Jun 1989 | Tsuyoshi Ohnishi | Simple blood test for diagnosing malignant hyperthermia |
| US4861790 * | 28 Oct 1987 | 29 Aug 1989 | Norwich Eaton Pharmaceuticals, Inc. | Use of azumolene for the treatment of malignant hyperthermia |
| US5462940 * | 3 Jun 1994 | 31 Oct 1995 | Norwich Eaton Pharmaceuticals, Inc. | 4-oxocyclic ureas useful as antiarrhythmic and antifibrillatory agents |
| US5691369 * | 7 Jun 1995 | 25 Nov 1997 | The Proctor & Gamble Company | Cardiovascular disorders |
| US5994354 * | 7 Jun 1995 | 30 Nov 1999 | The Procter & Gamble Company | Cyclic urethanes useful as antiarrhythmic and antifibrillatory agents |
| US7758890 | 1 Mar 2004 | 20 Jul 2010 | Lyotropic Therapeutics, Inc. | Treatment using dantrolene |
| US8110225 | 4 Mar 2010 | 7 Feb 2012 | Lyotropic Therapeutics, Inc. | Treatment using dantrolene |
| US8604072 | 19 Jan 2012 | 10 Dec 2013 | Lyotropic Therapeutics, Inc. | Treatment using dantrolene |
| US8685460 | 19 Jan 2012 | 1 Apr 2014 | Lyotropic Therapeutics, Inc | Treatment using dantrolene |
| EP2583670A1 | 5 Sep 2008 | 24 Apr 2013 | US Worldmeds LLC | Co-solvent compositions and methods for improved delivery of dantrolene therapeutic agents |
| WO2005013919A2 * | 1 Mar 2004 | 17 Feb 2005 | Lyotropic Therapeutics Inc | Treatment using dantrolene |
Granulation is the act or process of forming or crystallizing into grains.[1] Granules typically have a size range between 0.2 to 4.0 mm depending on their subsequent use.
Synonym “Agglomeration”: Agglomeration processes or in a more general term particle size enlargement technologies are great tools to modify product properties. Agglomeration of powders is widely used to improve physical properties like: wettability, flowability, bulk density and product appearance.
In the chemical industry, granulation refers to the act or process in which large objects are cut or shredded and remelted into granules or pellets.
In the pharmaceutical industry, granulation refers to the act or process in which primary powder particles are made to adhere to form larger, multiparticle entities called granules. It is the process of collecting particles together by creating bonds between them. Bonds are formed by compression or by using a binding agent. Granulation is extensively used in the manufacturing of tablets and pellets (or spheroids).
The granulation process combines one or more powder particles and forms a granule that will allow tableting or spheronization process to be within required limits. This way predictable and repeatable process is possible and quality tablets or pellets can be produced using tabletting or spheronization equipment.
Granulation is carried out for various reasons, one of those is to prevent the segregation of the constituents of powder mix. Segregation is due to differences in the size or density of the component of the mix. Normally, the smaller and/or denser particles tend to concentrate at the base of the container with the larger and/or less dense ones on the top. An ideal granulation will contain all the constituents of the mix in the correct proportion in each granule and segregation of granules will not occur.
Many powders, because of their small size, irregular shape or surface characteristics, are cohesive and do not flow well. Granules produced from such a cohesive system will be larger and more isodiametric, both factors contributing to improved flow properties.
Some powders are difficult to compact even if a readily compactable adhesive is included in the mix, but granules of the same powders are often more easily compacted. This is associated with the distribution of the adhesive within the granule and is a function of the method employed to produce the granule.
For example, if one were to make tablets from granulated sugar versus powdered sugar, powdered sugar would be difficult to compress into a tablet and granulated sugar would be easy to compress. Powdered sugar’s small particles have poor flow and compression characteristics. These small particles would have to be compressed very slowly for a long period of time to make a worthwhile tablet. Unless the powdered sugar is granulated, it could not efficiently be made into a tablet that has good tablet characteristics such as uniform content or consistent hardness.
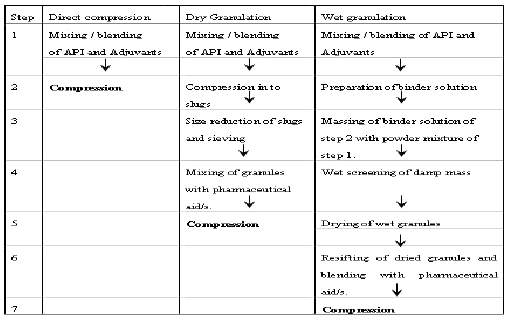
In pharmaceutical industry, two types of granulation technologies are employed, namely, wet granulation and dry granulation.
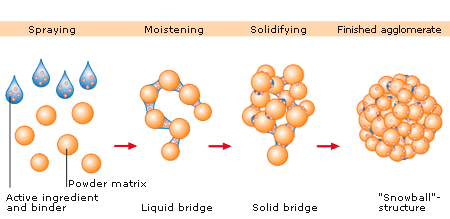
In wet granulation, granules are formed by the addition of a granulation liquid onto a powder bed which is under the influence of an impeller (in a High shear granulator, screws (in a twin screw granulator) [2] or air (in a fluidized bed granulator). The agitation resulting in the system along with the wetting of the components within the formulation results in the aggregation of the primary powder particles to produce wet granules.[2] The granulation liquid (fluid) contains a solvent which must be volatile so that it can be removed by drying, and be non-toxic. Typical liquids include water, ethanol and isopropanol either alone or in combination. The liquid solution can be either aqueous based or solvent based. Aqueous solutions have the advantage of being safer to deal with than solvents.
Water mixed into the powders can form bonds between powder particles that are strong enough to lock them together. However, once the water dries, the powders may fall apart. Therefore, water may not be strong enough to create and hold a bond. In such instances, a liquid solution that includes a binder (pharmaceutical glue) is required. Povidone, which is a polyvinyl pyrrolidone (PVP), is one of the most commonly used pharmaceutical binders. PVP is dissolved in water or solvent and added to the process. When PVP and a solvent/water are mixed with powders, PVP forms a bond with the powders during the process, and the solvent/water evaporates (dries). Once the solvent/water has been dried and the powders have formed a more densely held mass, then the granulation is milled. This process results in the formation of granules.
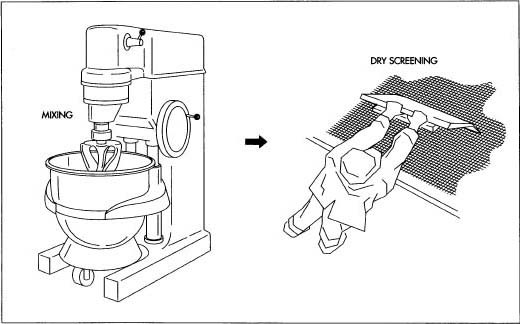
The process can be very simple or very complex depending on the characteristics of the powders, the final objective of tablet making, and the equipment that is available. In the traditional wet granulation method the wet mass is forced through a sieve to produce wet granules which is subsequently dried.
The dry granulation process is used to form granules without using a liquid solution because the product granulated may be sensitive to moisture and heat. Forming granules without moisture requires compacting and densifying the powders. In this process the primary powder particles are aggregated under high pressure. Sweying granulator or high shear mixer-granulator can be used for the dry granulation.
Dry granulation can be conducted under two processes; either a large tablet (slug) is produced in a heavy duty tabletting press or the powder is squeezed between two counter-rotating rollers to produce a continuous sheet or ribbon of materials (roller compactor, commonly referred to as a chilsonator).
When a tablet press is used for dry granulation, the powders may not possess enough natural flow to feed the product uniformly into the die cavity, resulting in varying degrees of densification. The roller compactor (granulator-compactor) uses an auger-feed system that will consistently deliver powder uniformly between two pressure rollers. The powders are compacted into a ribbon or small pellets between these rollers and milled through a low-shear mill. When the product is compacted properly, then it can be passed through a mill and final blend before tablet compression.


READ
March 2014, Volume 9, Issue 1, pp 16-37
http://link.springer.com/article/10.1007/s12247-014-9170-9
The wet granulation route of tablet manufacturing in a pharmaceutical manufacturing process is very common due to its numerous processing advantages such as enhanced powder flow and decreased segregation. However, this route is still operated in batch mode with little (if any) usage of an automatic control system. Tablet manufacturing via wet granulation, integrated with online/inline real time sensors and coupled with an automatic feedback control system, is highly desired for the transition of the pharmaceutical industry toward quality by design as opposed to quality by testing. In this manuscript, an efficient, plant-wide control strategy for an integrated continuous pharmaceutical tablet manufacturing process via wet granulation has been designed in silico. An effective controller parameter tuning strategy involving an integral of time absolute error method coupled with an optimization strategy has been used. The designed control system has been implemented in a flowsheet model that was simulated in gPROMS (Process System Enterprise) to evaluate its performance. The ability of the control system to reject the unknown disturbances and track the set point has been analyzed. Advanced techniques such as anti-windup and scale-up factor have been used to improve controller performance. Results demonstrate enhanced achievement of critical quality attributes under closed-loop operation, thus illustrating the potential of closed-loop feedback control in improving pharmaceutical tablet manufacturing operations.
……. CASE STUDY
http://www.madehow.com/Volume-4/Birth-Control-Pill.html
Oral contraceptives, or birth control pills, have been used by more than 60 million women worldwide, and are considered by many to be the most socially significant medical advance of the twentieth century. The birth control pill is a tablet taken daily by a woman to prevent pregnancy. The birth control pill does this by inhibiting the development of the egg in the woman’s ovary during her monthly menstrual cycle. During a woman’s menstrual cycle, a low estrogen level normally triggers the pituitary gland to send out a hormone that initiates development of an egg. The birth control pill releases enough synthetic estrogen to keep that hormone from being released during the monthly cycle.
A part is pasted. article talks of manufacturing process
please click link
Read more: http://www.madehow.com/Volume-4/Birth-Control-Pill.html#ixzz38GpZ5xQX
Read more: http://www.madehow.com/Volume-4/Birth-Control-Pill.html#ixzz38GpVEWtL
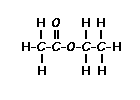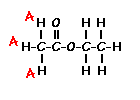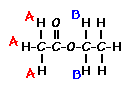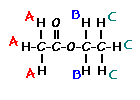
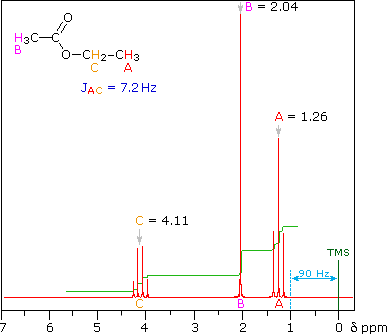
Place your arrow on above structure of Ethyl acetate………………It will flash
see label A,B,C
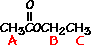
The intensity of the signal is proportional to the number of hydrogens that make the signal. Sometimes, NMR machines display signal intensity as an automatic display above the regular spectrum. (The exact number of hydrogens giving rise to each signal is sometimes also explicitly written above each peak, making our job a lot easier.) The intensity of the signal allows us to conclude that the more hydrogens there are in the same chemical environment, the more intense the signal will be.
We can get the following information from a 1H Nuclear Magnetic Resonance (NMR) structure:

The integrated intensity of a signal in a 1H NMR spectrum (does not apply to 13C NMR) gives a ratio for the number of hydrogens that give rise to the signal, thereby helping calculate the total number of hydrogens present in a sample.NMR machines can be used to measure signal intensity, a plot of which is sometimes automatically displayed above the regular spectrum. To show these integrations, a recorder pen marks a vertical line with a length that is proportional to the integrated area under a signal (sometimes referred to as a peak)– a value that is proportional to the number of hydrogens that are accountable for the signal. The pen then moves horizontally until another signal is reached, at which point, another vertical marking is made. We can manually measure the lengths by which the horizontal line is displaced at each peak to attain a ratio of hydrogens from the various signals. We can use this technique to figure out the hydrogen ratio when the number of hydrogens responsible for each signal is not written directly above the peak (look in the links section for an animation on how to manually find the ratio of hydrogens as described here).

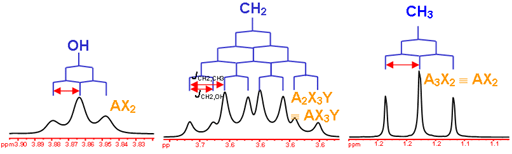

Now that we’ve seen how the signal intensity is directly proportionate to the number of hydrogens that give rise to that signal, it makes sense to conclude that the more hydrogens of one kind there are in a molecule (equivalent hydrogens, so in the same chemical environment), the more intense the corresponding NMR signal will be. Here’s above a model that may help clear up some of the uncertainties.


1.) True or False? The number of hydrogens determines the intensity of a signal.
ans…………False. The relative number of hydrogens determines the intensity of a signal. The signal given by the three hydrogens in CH3CH2CHCl2 will not have the same intensity as the three hydrogens in ClCH2OCH3.
2.) Give the number of signals, the chemical shift value for each signal, and the number of integrating hydrogens for CH3OCH2CH2OCH3
answer There are 2 signals. One is at 3.3 ppm (6 hydrogens); the other at 3.5 ppm (4 hydrogens).
3
Ethyl acetate contains 8 hydrogens and some of them are different from each other.
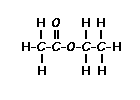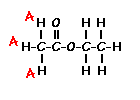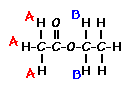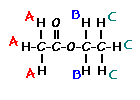 |
For example, those labeled A are attached to a carbon bonded to a carbonyl group and are different from the hydrogens labeled B which are bonded to a carbon attached to an oxygen atom. |
| You can check whether certain hydrogens are the same or equivalent by replacing each hydrogen with some group X and seeing if you generate the same compound. You should convince yourself that replacing each hydrogen labeled A by X gives you identical compounds which are all equivalent by a C-C bond rotation. If this is difficult to “see” look at this molecular model of ethyl acetate to see if you can convince yourself that all the hydrogens labeled A are the same. |
| The area under the NMR resonance is proportional to the number of hydrogens which that resonance represents. In this way, by measuring or integrating the different NMR resonances, information regarding the relative numbers of chemically distinct hydrogens can be found. Experimentally, the integrals will appear as a line over the NMR spectrum.Integration only gives information on the relative number of different hydrogens, not the absolute number. |
|
For ethyl acetate,
|
What ratio would you expect to see for the integrals for the hydrogens labeled A:B:C? |
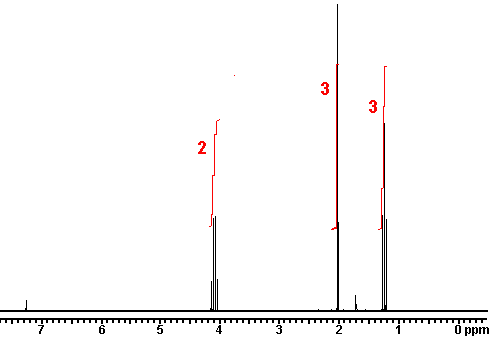
For ethyl ether,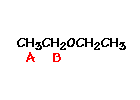 |
What ratio would you expect to see for the integrals for the hydrogens labeled A:B?3-2 |
For t-butyl acetate,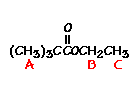 |
What ratio would you expect to see for the integrals for the hydrogens labeled A:B:C? |
4 6 6

Formula: C5H10O
C5H10O
Rule 2, omit O, gives C5H10
5 – 10/2 + 1 = 1 degree of unsaturation.
Look for 1 pi bond or aliphatic ring.


The band at 1727 indicates a carbonyl, probably an aldehyde; an aldehyde is also suggested by the band at 2719 which is likely the C-H stretch of the H-C=O group. The bands at 3000-2850 indicate C-H alkane stretches.

 n
n
Since the IR spectrum indicates an aldehyde, look for this functionality in the NMR spectrum. The aldehydic proton appears in the NMR from 9-10, usually as a small singlet.

The spectrum above shows a small singlet corresponding to one proton at 9.2 ppm, confirming that the compound is an aldehyde. Protons on the carbon adjacent to the aldehyde carbonyl will show up at 2-2.7 ppm; this is the triplet peak of 2 protons at 2.4 ppm on the above spectrum. Thus, so far we know that there is an aldehyde group next to a methylene group which is next to a carbon that has two hydrogens:

This accounts for 3 of the 5 carbons in the molecule. The un-colored hydrogens in the above structure could correspond to the peak of 2 hydrogens centered at 1.6 ppm; this peak is a pentet indicating that these protons are adjacent to carbons with a total of 4 hydrogens. The peak centered at 1.35 ppm has two hydrogens and is a sextet, indicating it is next to carbons that have a total of 5 hydrogens. Finally, the peak at 0.9 ppm has 3 hydrogens and is a triplet, indicating it is a methyl group adjacent to a carbon that has 2 hydrogens. Therefore, it looks like the molecule is a straight-chain of 5 carbons with the aldehyde group at one end:

Note that the closer a group is to the carbonyl function, the further downfield it is shifted. Here is how the NMR correlates to the structure:

MASS SPECTRUM