
Avibactam, sodium (2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl sulfonate,
Avibactam Sodium Salt (1)

Avibactam, sodium (2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl sulfonate,
Avibactam Sodium Salt (1)
D-2-Hydroxyglutarate (D-2HG) is frequently found in human brain cancers. Approximately 50–80% of grade II glioma patients have a high level of D-2HG production, which can lead to cancer initiation. In this study, a series of novel 5-hydroxy-2-methyl-4H-pyran-4-one derivatives were designed and synthesized as antiglioma agents, and their related structure–activity relationships are discussed. Among these novel compounds, 4a exhibited promising anti-proliferative activity against glioma HT1080 cells and U87 cells with an IC50 of 1.43 μM and 4.6 μM, respectively. Further studies found that the most active compound (4a) shows an 86.3% inhibitory rate against the intracellular production of D-2HG at 1 μM, and dramatic inhibitory effects, even at 1 μM on the colony formation and migration of U87 and HT1080 cells.

Structural modifications in the β-enamino diketone system allied to the Lewis acid carbonyl activator BF3 were strategically employed for this control. Also a one-pot method for the preparation of 3,5-disubstituted 4-hydroxymethyl-N-arylpyrazole derivatives from the β-enamino diketone and arylhydrazine substrates is described.

3-(Ethoxycarbonyl)-4-formyl-5-(4-nitrophenyl)-1-phenyl-1H-pyrazole (3a)
A sustainable procedure for the synthesis of various alkyl arylacetates from benzyl alcohols has been developed. With palladium as the catalyst and organic carbonates as the green solvent and in situ activator, benzyl alcohols were carbonylated in an efficient manner without any halogen additives.
Ethyl 2-phenylacetate
1H NMR (300 MHz, Chloroform-d) δ 7.32 – 7.08 (m, 5H), 4.08 (q, J = 7.1 Hz, 2H), 3.54 (s, 2H), 1.18 (t, J = 7.1 Hz, 3H).
13C NMR (75 MHz, CDCl3) δ 171.61, 134.17, 129.24, 128.54, 127.03, 60.85, 41.45, 14.18.
* Corresponding authors
Professor of Enzymology and Biophysical Chemistry
The construction of biocatalytic cascades for the production of chemical precursors is fast becoming one of the most efficient approaches to multi-step synthesis in modern chemistry. However, despite the use of low solvent systems and renewably resourced catalysts in reported examples, many cascades are still dependent on petrochemical starting materials, which as of yet cannot be accessed in a sustainable fashion. Herein, we report the production of the versatile chemical building block cinnamyl alcohol from the primary metabolite and the fermentation product L-phenylalanine. Through the combination of three biocatalyst classes (phenylalanine ammonia lyase, carboxylic acid reductase and alcohol dehydrogenase) the target compound could be obtained in high purity, demonstrable at the 100 mg scale and achieving 53% yield using ambient temperature and pressure in an aqueous solution. This system represents a synthetic strategy in which all components present at time zero are biogenic and thus minimises damage to the environment. Furthermore we extend this biocatalytic cascade by its inclusion in an L-phenylalanine overproducing strain of Escherichia coli. This metabolically engineered strain produces cinnamyl alcohol in mineral media using glycerol and glucose as the carbon sources. This study demonstrates the potential to establish green routes to the synthesis of cinnamyl alcohol from a waste stream such as glycerol derived, for example, from lipase treated biodiesel.
(R)-3-amino-3-(3-fluorophenyl)propanoic acid (1c) 1H NMR (CDCl3): δ 7.16-7.31 (m, 5H, ArH), 6.50-6.54 (d, 1H, J = 16 Hz, C=CH), 6.23-6.30 (dt, 1H, J = 16, 8 Hz, C=CHCH2 ), 4.21-4.23 (dd, 2H, J = 8, 4 Hz, C=CHCH2); 13C NMR (CDCl3): 136.70, 131.09, 128.60, 128.54, 127.69, 126.48, 63.65.
////////////cinnamyl alcohol, biocatalytic, metabolic engineering
The sustainable introduction of nitrogen moieties in the form of nitrile or amide groups in functionalized molecules is of fundamental interest because nitrogen-containing motifs are found in a large number of life science molecules, natural products and materials. Hence, the synthesis and functionalization of nitriles and amides from easily available starting materials using cost-effective catalysts and green reagents is highly desired. In this regard, herein we report the nanoscale iron oxide-catalyzed environmentally benign synthesis of nitriles and primary amides from aldehydes and aqueous ammonia in the presence of 1 bar O2 or air. Under mild reaction conditions, this iron-catalyzed aerobic oxidation process proceeds to synthesise functionalized and structurally diverse aromatic, aliphatic and heterocyclic nitriles. Additionally, applying this iron-based protocol, primary amides have also been prepared in a water medium.
1H NMR (300 MHz, Chloroform-d) δ 7.17 – 6.96 (m, 2H), 6.93 – 6.70 (m, 1H), 4.33 – 4.11 (m, 4H). 13C NMR (75 MHz, Chloroform-d) δ 147.75 , 143.80 , 125.87 , 121.21 , 118.91 , 118.25 , 104.38 , 64.59 , 64.12 . Off white solid
cas 19102-07-9
MP
| Melting Point, °C | ||
|---|---|---|
| 105 – 106
Tetrahedron, 2015, vol. 71, 29, p. 4883 – 4887 |
NMR PREDICTS
1H NMR
13C NMR PREDICT
More…………….
Journal of the American Chemical Society, 2001, vol. 123, 49, p. 12202 – 12206
More………….
RSC Advances, 2013, vol. 3, 44, p. 22389 – 22396
http://www.rsc.org/suppdata/ra/c3/c3ra44386h/c3ra44386h.pdf
MORE……..
Organic Letters, 2017, vol. 19, 12, p. 3095 – 3098
http://pubs.acs.org/doi/suppl/10.1021/acs.orglett.7b01199/suppl_file/ol7b01199_si_001.pdf
2,3-Dihydrobenzo[b][1,4]dioxine-6-carbonitrile (Scheme 1, 2n) According to the general procedure A, the reaction of 1n (0.20 mmol), zinc cyanide (2.0 equiv), PCyPh2 (0.20 equiv) and Pd(OAc)2 (0.05 equiv) in dioxane (0.25 M) for 16 h at 150 °C, afforded after work-up and chromatography the title compound in 75% yield (24.2 mg). White solid. 1H NMR (500 MHz, CDCl3) δ 7.17-7.11 (m, 2H), 6.91 (d, J = 8.1 Hz, 1H), 4.32-4.31 (m, 2H), 4.30- 4.26 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 147.84, 143.91, 126.04, 121.37, 119.01, 118.37, 104.62, 64.71, 64.24.
//////////////
A sulfurative self-condensation method for constructing thiophenes 2 by a reaction between ketones 1 and elemental sulfur is reported. This reaction, which is catalyzed by anilines and their salts with strong acids, starts from readily available and inexpensive materials, and releases only water as a by-product.
2,4-Di-p-tolylthiophene (2b)2
2 M. Arisawa, T. Ichikawa, and M. Yamaguchi, Chem. Commun. 2015, 51, 8821
Eluent heptane:toluene 9:1. 190 mg, 72%.
1 H NMR (300 MHz, CDCl3) δ 7.60-7.54 (m, 5H), 7.34 (s, 1H), 7.27-7.23 (m, 4H), 2.42 (s, 6H).
13C NMR (75 MHz, CDCl3) δ 145.3, 143.3, 137.8, 137.2, 133.5, 131.9, 129.9, 129.8, 126.5, 126.0, 122.1, 118.9, 21.5, 21.5.


CNRS Research Associate CR1 ( ORCID , ResearchGate )
ICSN-CNRS Bât. 27
1, avenue de la Terrasse
91190 Gif-sur-Yvette France
thanh-binh.nguyen_at_cnrs.fr
+33 1 69 82 45 49
![]() Education and work experience2015: Habilitation to Direct Research (HDR)
Education and work experience2015: Habilitation to Direct Research (HDR)
2011 – present: CNRS research associate at ICSN – Paris-Saclay University
2009 – 2011: Post-doctoral Fellow at ICSN (Dr. Françoise Guéritte and Dr. Qian Wang)
2003 – 2006: Ph.D. student at the UCO2M Organic Synthesis Laboratory (University of Maine, Le Mans, France, Dr. Gilles Dujardin, Dr. Arnaud Martel, Professor Robert Dhal)
![]() Research Interests
Research Interests
Green chemistry (Atom, step and redox economic transformation), green synthetic tools: O2, S8, photochemistry, iron catalyst
Elemental sulfur as a synthetic tool (building block, oxidant, reductant, catalyst)
Iron-sulfur catalysts
Heterocycle synthesis
![]() Scientific Communications
Scientific Communications
47 publications
![]() Selected recent publications ( complete list )
Selected recent publications ( complete list )
[1] Adv. Synth. Catal. 2017 , 359 , 1106.
[2] Asian J. Org. Chem. 2017 , 6 , 477.
[3] Org. Lett. 2016 , 18 , 2177.
[4] Org. Process Res. Dev. 2016 , 20 , 319.
[5] Angew. Chem. Int. Ed. 2014 , 53 , 13808.
[6] J. Am. Chem. Soc. 2013 , 135 , 118.

///////////
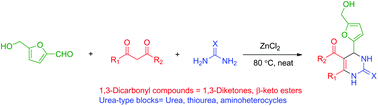
The use of the renewable platform molecule 5-hydroxymethylfurfural (HMF) in the multi-component Biginelli reaction has been investigated. Multicomponent reactions (MCR) using HMF offer straightforward access to novel fine chemicals. However, the peculiar reactivity and lower stability of HMF have limited its use in such strategies. In this paper, we report our results on the use of HMF in 3-component Biginelli reactions, leading in one single step to a series of functionalized dihydropyrimidinones obtained in moderate to good yields, with a broad substrate scope of 1,3-dicarbonyl compounds and urea building blocks. This is the first report on the use of HMF in this reaction. The CH2OH motif found in HMF provides useful functionalization for the target molecules, which cannot be offered by simpler aldehydes such as furfural.
5-Acetyl-4-[5’-(hydroxymethyl)furan-2’-yl]-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4a):
Reaction time: 8 h; Global yield: 86%; (78% yield after simple filtration + additional 8% yield after purification of the filtrate by column chromatography).
1H NMR (400 MHz, DMSO-d6) δ 9.22 (d, 1H, J = 1.2 Hz, H1), 7.88 (dd, 1H, J = 3.4, 1.2 Hz, H3), 6.16 (d, 1H, J = 3.1 Hz, H4’), 6.03 (d, 1H, J = 3.1 Hz, H3’), 5.27 (d, 1H, J = 3.4 Hz, H4), 5.18 (t, 1H, J = 5.6 Hz, OH), 4.33 (d, 2H, J = 5.6 Hz, CH2), 2.25 (s, 3H, CH3-C6), 2.17 (s, 3H, CH3CO).
13C NMR (100 MHz, DMSO-d6) δ 193.9 (COCH3), 155.1, 154.9 (C2’, C5’), 152.4 (C2), 149.0 (C6), 107.7 (C4’), 107.1 (C5), 106.3 (C3’), 55.7 (CH2OH), 47.9 (C4), 30.0 (CH3CO), 19.0 (CH3-C6).
HRMS (ESI) m/z: Calcd for [M+Na]+ C12H14N2NaO4 273.0846; Found 273.0850.



The present work shows that it is possible to ring-open cyclic carbonates with unprotected amino acids in water. Fine tuning of the reaction parameters made it possible to suppress the degree of hydrolysis in relation to aminolysis. This enabled the synthesis of functionally dense carbamates containing alkenes, carboxylic acids, alcohols and thiols after short reaction times at room temperature. When Glycine was used as the nucleophile in the ring-opening with four different five membered cyclic carbonates, containing a plethora of functional groups, the corresponding carbamates could be obtained in excellent yields (>90%) without the need for any further purification. Furthermore, the orthogonality of the transformation was explored through ring-opening of divinylenecarbonate with unprotected amino acids equipped with nucleophilic side chains, such as serine and cysteine. In these cases the reaction selectively produced the desired carbamate, in 70 and 50% yield respectively. The synthetic design provides an inexpensive and scalable protocol towards highly functionalized building blocks that are envisioned to find applications in both the small and macromolecular arena.


Chief Technology Officer
Dr. Eric V. Johnston obtained his Master of Science degree in 2008 at the Department of Organic Chemistry, Stockholm University, Sweden. In the same year, he started his graduate studies under the supervision of Prof. Jan-Erling Bäckvall. During his PhD, he worked on the development of new homogeneous and heterogeneous transition-metal catalysts.
After receiving his PhD in 2012, he joined Prof. Samuel J. Danishefskys research group at Memorial Sloan-Kettering Cancer Center, New York, USA as a postdoctoral fellow supported by The Swedish Research Council. Here he was engaged in the total chemical synthesis of glycolsylated proteins that play important roles in modern cancer treatment.
In 2014 he returned to the Department of Organic Chemistry at Stockholm University to establish his own group. The goal of his research is to contribute new advances to the strategy and methodology for the preparation of synthetic macromolecules such as proteins, glycopeptides, sequence and length-controlled polymers. He is also a Co-Supervisor for Prof. Björn Åkermarks research group, which aims at studying and developing new homogeneous, as well as heterogeneous, water oxidation catalysts.
A practical synthesis of 2,3-dihydro-1,5-benzothiazepines
*Corresponding authors
Domenico Albanese received his Ph.D. degree in 1993 with Prof. Dario Landini working on phase transfer catalysis. After short stays at Imperial College London and the Technical University of Denmark, he gained a permanent position at the Università degli Studi di Milano, where he was appointed associate professor in 2008. His research interests include novel developments of phase-transfer catalysis, green chemistry and the development of new environmentally friendly antifouling agents.

2,3-Dihydro-1,5-benzothiazepines have been obtained through a domino process involving a Michael addition of 2-aminothiophenols to chalcones, followed by in situ cyclization. Up to 98% chemical yields have been obtained at room temperature under essentially neutral conditions by using hexafluoro-2-propanol as an efficient medium.