An efficient synthetic approach for the synthesis of α-pyrones via Baeyer–Villiger-type oxidation of α-iodocyclopentenones through a catalyst- and additive-free system using air as an environmentally benign oxidant is described. The reaction exhibits excellent functional group compatibility and provides a simple and efficient protocol for the construction of highly functionalized α-pyrones under mild reaction conditions.
Towards cleaner PolarClean: efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate
Abstract
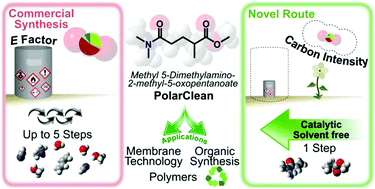
As a result of recent efforts in green solvent selection, methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, sold under the brand name Rhodiasolv PolarClean, has received considerable scientific and industrial attention as a possible non-toxic replacement for common polar aprotic solvents. However, the multicomponent nature and multi-step synthesis of this solvent remains an obstacle for its more widespread use and niche applications. In this work, a retrosynthetic approach was taken to identify novel shorter synthetic routes in alignment with green chemistry principles. High purity methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate was obtained in novel single-step reactions via two different base-catalysed Michael additions from readily available building blocks. The more advanced synthetic route shows great potential owing to the swift (30 min), solvent-free reaction and catalytic amounts of base (<6.5 mol%). Green metrics analysis, including Atom Economy, Complete E factor, Carbon Intensity and hazard analysis found the new synthesis to be more sustainable than the patented routes. Application of this green solvent was demonstrated for the first time for O– and N-arylation in SNAr reaction with solvent recovery with similar or superior yields compared to other green solvents. Moreover, broad opportunities for this green solvent in membrane science were identified, where the use of conventional, toxic polar aprotic solvents in large quantities is unavoidable. Important practical solvent properties and parameters such as dielectric constant, solubility parameters, solvent miscibility and NMR residual shifts have been determined to facilitate the uptake of methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate as a green solvent.
Towards cleaner PolarClean: efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate
////////
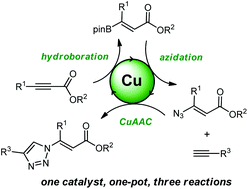
Triple copper catalysis for the synthesis of vinyl triazoles
DOI: 10.1039/C9GC01066A, Communication
Herein, we report our studies on the synthesis of vinyl 1,2,3-triazoles through a one-pot sequence, enabled by the same copper catalyst, of three different reactions: hydroboration involving an alkyne, azidation for a vinyl boronate and azide–alkyne cycloaddition.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
Triple copper catalysis for the synthesis of vinyl triazoles
Abstract
Herein, we report our studies on the synthesis of vinyl 1,2,3-triazoles through a one-pot sequence, enabled by the same copper catalyst, of three different reactions: hydroboration involving an alkyne, azidation for a vinyl boronate and azide–alkyne cycloaddition.
///////copper catalysis, vinyl triazoles
A solvent-free catalytic protocol for the Achmatowicz rearrangement
Abstract
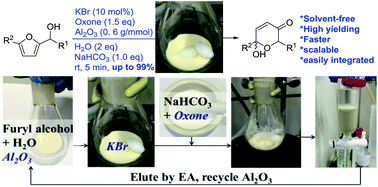
Reported here is the development of an environmentally friendly catalytic (KBr/oxone) and solvent-free protocol for the Achmatowicz rearrangement (AchR). Different from all previous methods is that the use of chromatographic alumina (Al2O3) allows AchR to proceed smoothly in the absence of any organic solvent and therefore considerably facilitates the subsequent workup and purification with minimal environmental impacts. Importantly, this protocol allows for scaling up (from milligram to gram), recycling of the Al2O3, and integrating with other reactions in a one-pot sequential manner.
A solvent-free catalytic protocol for the
Achmatowicz rearrangement
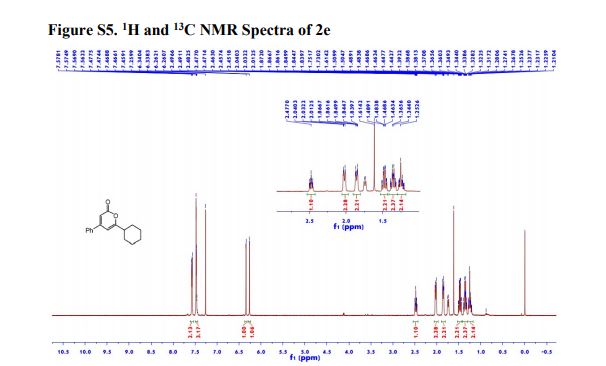
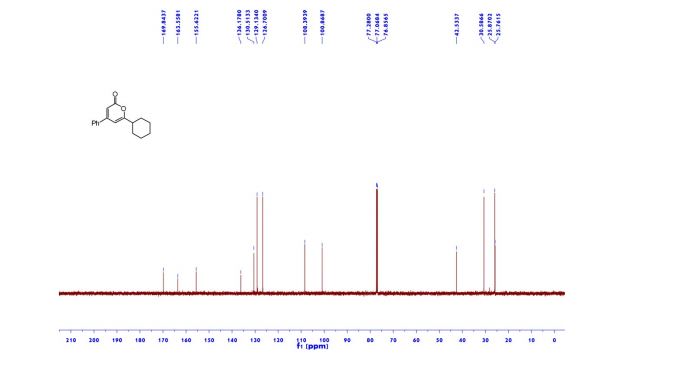
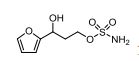
1n: colorless oil, 0.33 g, 73% yield for 2 steps.
1H-NMR (400 MHz, DMSO) δ: 7.59–7.58 (m, 1H), 7.45 (s, 2H), 6.40 (dd, J = 3.2, 1.8 Hz, 1H), 6.29 (d, J = 3.2 Hz, 1H), 5.49 (s, 1H), 4.74–4.60 (m, 1H), 4.18–4.07 (m, 2H), 2.09–2.04 (m, 2H).
13C-NMR (100 MHz, DMSO) δ: 157.6, 142.4, 110.7, 106.1, 66.5, 62.8, 35.2. IR (KBr) 3282.9, 2928.7, 1627.4, 1562.5, 1353.8, 1174.6, 1074.0, 999.7, 918.4, 742.8 cm-1 ;
HRMS (CI+ ) (m/z) calcd. for C7H11NO5S [M]+ 221.0352; found 221.0354.
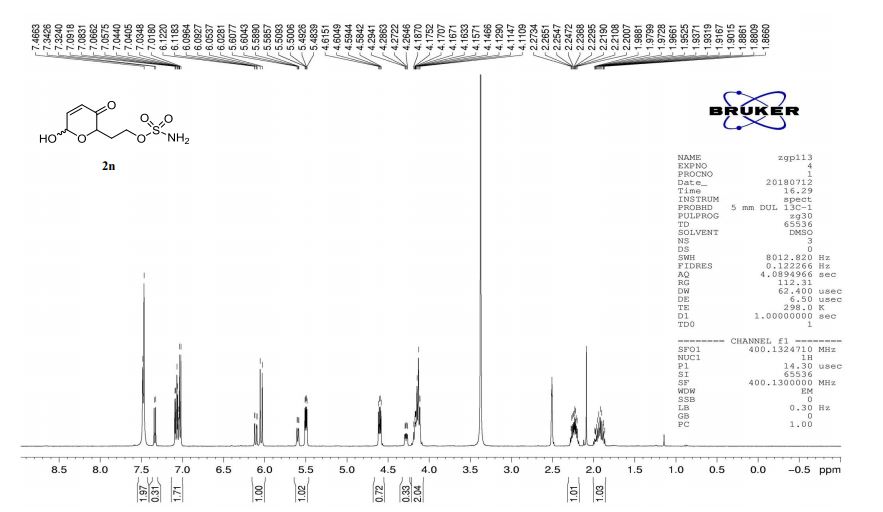
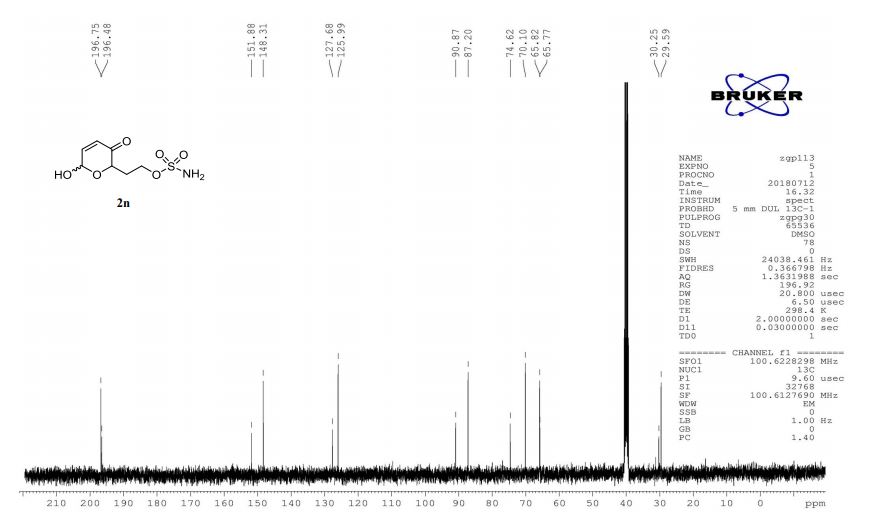
2n (EtOAc/hexane = 3:1):colorless oil (dr 7:3), 46 mg, 97%.
1H-NMR (400 MHz, DMSO) δ: 7.48–7.47 (m, 2H), 7.34–7.02 (m, 2H), 6.12–6.03 (m, 1H), 5.61–5.48 (m, 1H), 4.60 (dd, J = 8.3, 4.1 Hz, 0.7H), 4.28 (ddd, J = 8.8, 4.0, 1.3 Hz, 0.3H), 4.20–4.11 (m, 2H), 2.27–2.20 (m, 1H), 1.97–1.86 (m, 1H).
13C-NMR (100 MHz, DMSO) δ: 196.7, 196.5, 151.9, 148.3, 127.7, 126.0, 90.9, 87.2, 74.6, 70.1, 65.8, 65.8, 30.3, 29.6. IR (KBr) 3370.4, 2987.0, 1689.5, 1364.3, 1268.0, 1178.4, 1023.3, 928.3, 755.1 cm-1 ;
HRMS (CI+ ) (m/z) calcd. for C7H11NO6S [M]+ 237.0302; found 237.0315.
////////////////Achmatowicz rearrangement
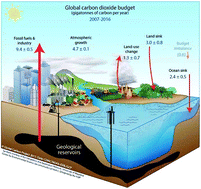
A call to (green) arms: a rallying cry for green chemistry and engineering for CO2 capture, utilisation and storage
A call to (green) arms: a rallying cry for green chemistry and engineering for CO2 capture, utilisation and storage
Abstract
Chemists, engineers, scientists, lend us your ears… Carbon capture, utilisation, and storage (CCUS) is among the largest challenges on the horizon and we need your help. In this perspective, we focus on identifying the critical research needs to make CCUS a reality, with an emphasis on how the principles of green chemistry (GC) and green engineering can be used to help address this challenge. We identify areas where GC principles can readily improve the energy or atom efficiency of processes or reduce the environmental impact. Conversely, we also identify dilemmas where the research needs may be at odds with GC principles, and present potential paths forward to minimise the environmental impacts of chemicals and processes needed for CCUS. We also walk a different path from conventional perspectives in that we postulate and introduce potential innovative research directions and concepts (some not yet experimentally validated) in order to foster innovation, or at least stoke conversation and question why certain approaches have not yet been attempted. With elements of historical context, technological innovation, critical thinking, and some humour, we issue a call to arms and hope you may join us in this fight.

David Heldebrant
PO Box 999
Richland, WA 99352
/////////
Asymmetric Organocatalysis in Drug Development—Highlights of Recent Patent Literature
Enantioselective organocatalytic reactions published in the recent patent literature are highlighted in this review and include inter- and intramolecular phase-transfer conjugate additions catalyzed by quaternized cinchona alkaloids, a Diels–Alder reaction catalyzed by oxazaborolidine complexes, asymmetric Betti reactions, the Lonza synthesis of l-carnitine, and several Corey–Bakshi–Shibata reductions.
Asymmetric Organocatalysis in Drug Development—Highlights of Recent Patent Literature
https://pubs.acs.org/doi/10.1021/acs.oprd.8b00096
///////////////Asymmetric Organocatalysis
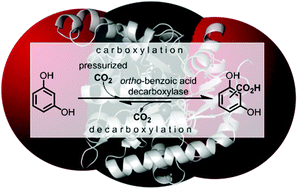
Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol
DOI: 10.1039/C8GC00008E, Communication
 Open Access
Open Access
 This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Utilization of gaseous carbon dioxide as a C1-building block in the biocatalytic ortho-carboxylation of a phenol.
The content of this RSS Feed (c) The Royal Society of Chemistry
Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol
Abstract
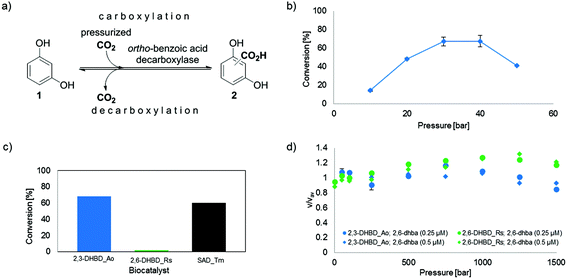
The utilization of gaseous carbon dioxide instead of bicarbonate would greatly facilitate process development for enzyme catalyzed carboxylations on a large scale. As a proof-of-concept, 1,3-dihydroxybenzene (resorcinol) was carboxylated in the ortho-position using pressurized CO2 (∼30–40 bar) catalyzed by ortho-benzoic acid decarboxylases with up to 68% conversion. Optimization studies revealed tight pH-control and enzyme stability as the most important determinants.
 |
||
Fig. 1 (a) Enzyme-catalyzed de/carboxylation of resorcinol (1). Carboxylated product 2 is a mixture of regio-isomeric 2,6- (2,6-dhba, 2a) and 2,4-dihydroxybenzoic acid (2,4-dhba, 2b) with a ratio of 3![[thin space (1/6-em)]](http://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](http://www.rsc.org/images/entities/char_2009.gif) 4;10b (b) CO2 pressure dependence of the carboxylation of 1 using 2,3-DHBD_Ao; (c) carboxylation activity of decarboxylases with CO2 (30 bar) using 1 as a substrate; (d) stopped-flow measurements of the decarboxylation of 2,6-dhba (2a) with pressure-pretreated (≤1.5 kbar) 2,3-DHBD_Ao and 2,6-DHBD_Rs. 4;10b (b) CO2 pressure dependence of the carboxylation of 1 using 2,3-DHBD_Ao; (c) carboxylation activity of decarboxylases with CO2 (30 bar) using 1 as a substrate; (d) stopped-flow measurements of the decarboxylation of 2,6-dhba (2a) with pressure-pretreated (≤1.5 kbar) 2,3-DHBD_Ao and 2,6-DHBD_Rs. |
||
Conclusion
In summary, we have demonstrated that pressurized carbon dioxide can be used directly as a carboxylating agent in the enzyme catalyzed o-carboxylation of a phenol as an alternative to the high concentration of bicarbonate. Two enzyme candidates (2,3-DHBD_Ao and SAD_Tm) readily accepted the alternative CO2 source for the carboxylation of the model substrate resorcinol. In contrast, 2,6-DHBD_Rs was inapplicable under CO2 pressure due to irreversible inactivation, which was correlated to a decrease in pH caused by the dissociation of H2CO3.
Overall, the use of pressurized CO2 gas significantly improves the efficiency of biocatalytic carboxylations and facilitates downstream-processing of this benign and sustainable approach in using CO2 as a carbon feedstock for the synthesis of organic acids.

DOCTORAL STUDENT,
Katharina Plasch
tel: +43-316-380-8646
e-mail:katharina.plasch(at)uni-graz.at

Kurt Faber
Institute of Chemistry – Organic & Bioorganic Chemistry
Karl Franzens Universität Graz
Heinrichstrasse 28
8010 Graz
e-Mail: kurt.faber@uni-graz.at
phone: +43 316 380 5332
fax: +43 316 380 9840
web: http://biocatalysis.uni-graz.at
Kurt Faber, born 1953 in Klagenfurt (Carinthia/Austria), studied chemistry at the University of Graz, where he received his PhD in 1982. After a post-doctoral fellowship from 1982 to 1983 in St. John’s (Canada) he continued his career at the University of Technology in Graz, where he became associate professor in 1997. The following year he was appointed full professor at the University of Graz, where he since heads a research group devoted to the use of biocatalysts for the transformation of non-natural compounds. He was a visiting scientist at University of Tokyo (1987/1988), Exeter University (1990), University of Trondheim (1994), Stockholm University (2001) and University of Minnesota (2005).

Silvia M. Glueck
Silvia M. Glueck, born 1973 in Bruck/Mur (Austria), studied Chemistry at the University of Graz, where she received her PhD under the supervision of Prof. K. Faber in 2004. From 2005 to 2006 she worked as a post-doctoral fellow with Prof. N. J. Turner at the University of Edinburgh and the University of Manchester (MIB). In 2007 she returned to the University of Graz to join Prof. W. Kroutil, and in 2008 she became Research Scientist at the Research Centre Applied Biocatalysis in the group of Prof. K. Faber. Her research interests are focusing on biocatalysis, molecular biology and organic synthesis.

THE ELK CREW
We keep science in motion…
RESEARCHERS
Emmanuel Cigan • Elisabeth Eger • Judith Farnberger • Michael Fuchs • Somayyeh Gandomkar • Silvia Glueck • Christopher Grimm • Barbara Grischek • Mélanie Hall •Valentina Jurkaš • Lucas Hammerer • Barbara Larissegger • Haifeng Liu • Lía Martínez Montero • Stefan Payer • Philipp Petermeier • Mathias Pickl • Katharina Plasch • Jakob Pletz • Hannah Pollak • Silvan Poschenrieder • Tamara Reiter • Luca Schmermund • Joerg Schrittwieser • Erika Tassano • Gábor Tasnádi • Stefan Velikogne • Christoph Winkler •
//////////
Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol
K. Plasch, G. Hofer, W. Keller, S. Hay, D. J. Heyes, A. Dennig, S. M. Glueck and K. Faber, Green Chem., 2018, 20, 1754
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. Material from this article can be used in other publications provided that the correct acknowledgement is given with the reproduced material.
Reproduced material should be attributed as follows:
- For reproduction of material from NJC:
[Original citation] – Published by The Royal Society of Chemistry (RSC) on behalf of the Centre National de la Recherche Scientifique (CNRS) and the RSC. - For reproduction of material from PCCP:
[Original citation] – Published by the PCCP Owner Societies. - For reproduction of material from PPS:
[Original citation] – Published by The Royal Society of Chemistry (RSC) on behalf of the European Society for Photobiology, the European Photochemistry Association, and RSC. - For reproduction of material from all other RSC journals:
[Original citation] – Published by The Royal Society of Chemistry.
The Green ChemisTREE: 20 years after taking root with the 12 principles
DOI: 10.1039/C8GC00482J, Critical Review
A broad overview of the achievements and emerging areas in the field of Green Chemistry.
The Green ChemisTREE: 20 years after taking root with the 12 principles
Abstract
The field of Green Chemistry has seen many scientific discoveries and inventions during the 20 years since the 12 Principles were first published. Inspired by tree diagrams that illustrate diversity of products stemming from raw materials, we present here the Green ChemisTREE as a showcase for the diversity of research and achievements stemming from Green Chemistry. Each branch of the Green ChemisTREE represents one of the 12 Principles, and the leaves represent areas of inquiry and development relevant to that Principle (branch). As such, in this ‘meta-review’, we aim to describe the history and current status of the field of Green Chemistry: by exploring activity within each Principle, by summarizing the benefits of Green Chemistry through robust examples, by discussing tools and metrics available to measure progress towards Green Chemistry, and by outlining knowledge gaps, opportunities, and future challenges for the field.
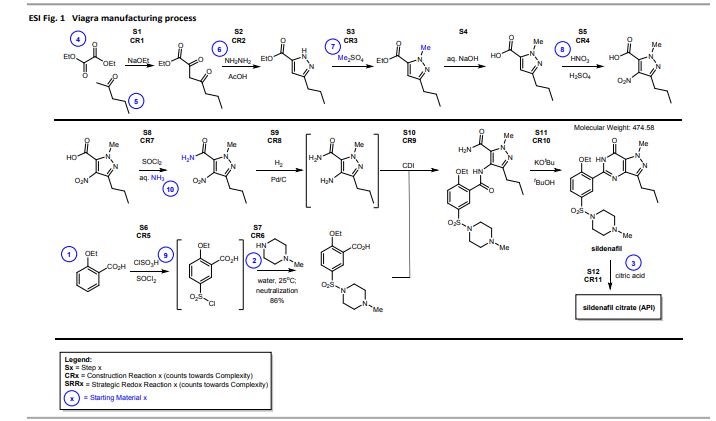
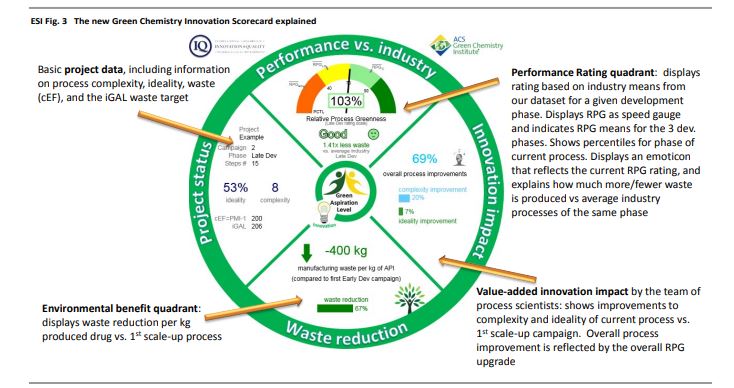
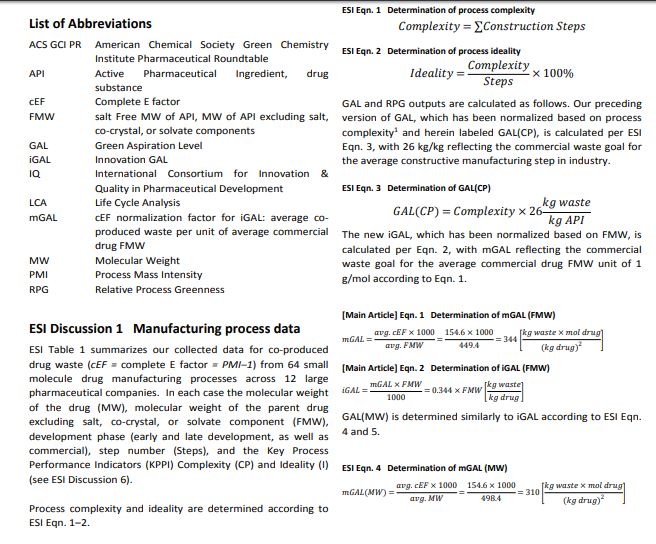
Inspiring process innovation via an improved green manufacturing metric: iGAL
DOI: 10.1039/C8GC00616D, Communication
Abstract
Following our goal to devise a unified green chemistry metric that inspires innovation in sustainable drug manufacturing across the pharmaceutical industry, we herein disclose joint efforts by IQ, the ACS GCI PR and academia, leading to the significantly improved ‘innovation Green Aspiration Level’ (iGAL) methodology. Backed by the statistical analysis of 64 drug manufacturing processes encompassing 703 steps across 12 companies, we find that iGAL affords an excellent proxy for molecular complexity and presents a valuable molecular weight-based ‘fixed’ goal. iGAL thereby accurately captures the impact of green process inventiveness and improvements, making it a useful innovation-driven green metric. We conclude by introducing the comprehensive, yet easy-to-use and readily adaptable Green Chemistry Innovation Scorecard web calculator, whose graphical output clearly and effectively illustrates the impact of innovation on waste reduction during drug manufacture.
Following our goal to devise a unified green chemistry metric that inspires innovation in sustainable drug manufacturing across the pharmaceutical industry, we herein disclose joint efforts by IQ, the ACS GCI PR and academia, leading to the significantly improved ‘innovation Green Aspiration Level’ (iGAL) methodology. Backed by the statistical analysis of 64 drug manufacturing processes encompassing 703 steps across 12 companies, we find that iGAL affords an excellent proxy for molecular complexity and presents a valuable molecular weight-based ‘fixed’ goal. iGAL thereby accurately captures the impact of green process inventiveness and improvements, making it a useful innovation-driven green metric. We conclude by introducing the comprehensive, yet easy-to-use and readily adaptable Green Chemistry Innovation Scorecard web calculator, whose graphical output clearly and effectively illustrates the impact of innovation on waste reduction during drug manufacture.

Director – Process Research & Global External Chemistry Management
Boehringer Ingelheim
Ange Zhou
California State University, East Bay

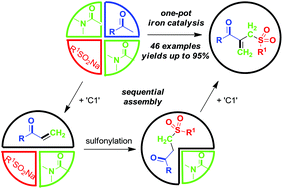
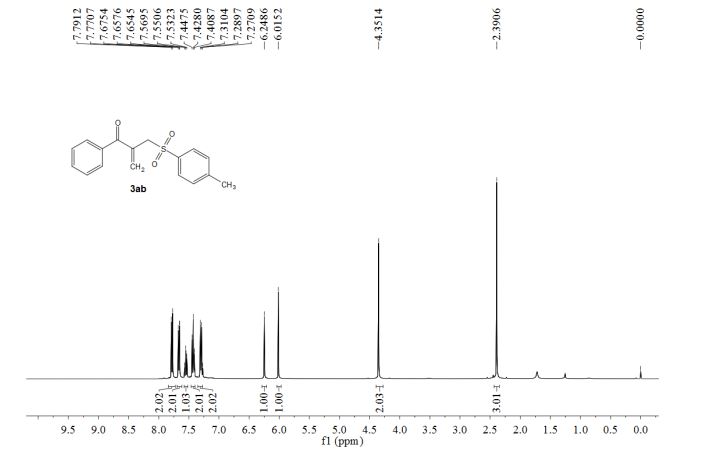
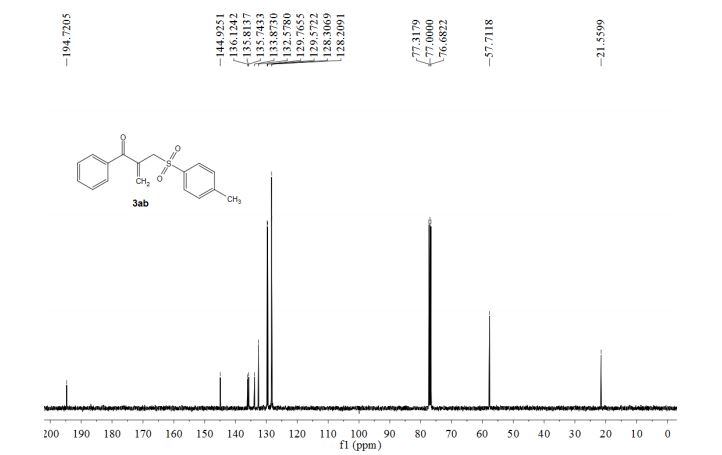
Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly
Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly
DOI: 10.1039/C7GC03719H, Communication
A three starting material four component reaction (3SM-4CR) strategy is described to prepare [small beta]-acyl allylic sulfones from methyl ketones, sodium sulfinates and dimethylacetamide (DMA) in an iron-catalyzed oxidative system.
Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly
Abstract
A three starting material four component reaction (3SM-4CR) strategy is described to prepare β-acyl allylic sulfones from methyl ketones, sodium sulfinates and dimethylacetamide (DMA) in an iron-catalyzed oxidative system. In this process, DMA was used as a dual synthon to provide two carbons. A broad range of functional groups were tolerated in this reaction system.






















 Paul T. Anastas,
Paul T. Anastas,