
Anti-Aging Secret
A substance found in pomegranate fruit proved an effective anti-aging agent

CREDIT http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.4132.html
CREDIT ChemistryViews
/////////////pomegranate fruit, anti-aging agent

A substance found in pomegranate fruit proved an effective anti-aging agent

CREDIT http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.4132.html
CREDIT ChemistryViews
/////////////pomegranate fruit, anti-aging agent

| Diabetes Nephropathy, a chronic metabolic complication of diabetes mellitus, is characterized by elevated levels of serum glucose,creatinine, urea and uric acid in addition to abnormal histopathological changes in kidney. In the recent past, many antidiabetic agents are introduced; still the diabetes and the related nephropathy complication continue to be a major medical problem, not only in developed countries but also in developing countries. Not with standing much research work, the diabetic kidney damages are increasing rapidly and patients with diabetes kidney failure undergo either painful dialysis or kidney transplantation [1] which is both costly and harmful. More and more interest is now growing about plant use as an alternative therapy for protecting kidney damage in patients with diabetes mellitus. Reactive oxygen species (ROS) have been widely implicated in the pathogenicity of diabetes mellitus and its nephropathy. A number of clinical studies suggest that the antioxidants in medicinal plants are key factors in reducing the incidence of diabetic nephropathy. Traditional medicines and extracts from medicinal plants with antioxidant potential have been extensively used as alternative medicine for better control and management of diabetes nephropathy [2]. However, searching for new antidiabetic drugs with nephroprotective properties from natural plants is currently very important. |
| Amaranthus hybridus L. (Amaranthaceae) commonly known as ‘Cheera’ in Malayalam, is an erect branched annual herb distributed throughout tropical and temperate regions of India as a common weed in the agricultural fields and wastelands. In traditional medicinal system different parts of the plant Amaranthus hybridus (A. hybridus) have been mentioned to be useful in a variety of diseases. Traditionally, the plant has been used in treating dysentery, diarrhoea, ulcers and hemorrhage of the bowel due to its astringent property [3–5]. In southern India, the leaves are used in folk medicine for the treatment of diabetes. Leaves possess antibacterial effect, cleansing effect and also help to reduce tissue swelling [5]. In Nigeria, A. hybridus leaves combined with condiments are used to prepare soup [6–8]. In Congo, their leaves are eaten as spinach or green vegetables [6,9]. These leaves boiled and mixed with a groundnut sauce are eaten as salad in Mozambique and in West Africa [10,11]. The Amaranthus species contains amaranthine, quercetin, and kaempferol glycosides [12].A. hybridus leaves are used as an antidote for snake and scorpion bite [13,14]. |
| Amaranthus species were of great importance in pre-Colombian American people’s diets [15] and A. cruentus and A. hybridus have a high nutritional value [16] (Fernand et al.). The consumption of A. cruentus products is advised for patients with celiac disease and, therefore, also for diabetic persons [17]. A. hybridus has been used traditionally for the treatment of liver infections and knee pain and for its laxative, diuretic, and cicatrisation properties [16]. |
| Furthermore, recent studies established theantihyperglycemic activities of other species of Amaranthus genus as A. spinosus [18] and A. viridis [19,20]. However, based on the literature survey, there is no scientific report proving the anti-hyperglycemic efficacy of this particular species. Therefore, the current study was designed to evaluate the nephroprotective activity of Amaranthus hybridus in STZ induced diabetic rats. |
| Balasubramanian T* and Karthikeyan M | |
| Department of Pharmacology, Al Shifa College of Pharmacy, Kerala, India | |
| Corresponding Author : | Dr. Thirumalaiswamy Balasubramanian Department of Pharmacology Al Shifa College of Pharmacy Poonthavanam Post, Kizhattur Village Perinthalmanna, Malappuram Dist Kerala-679 325, India Tel: +919544496752 E-mail: tbaluanandhi@gmail.com |
| Received December 29, 2015; Accepted January 07, 2016; Published January 14, 2016 | |
| Citation: Balasubramanian T and Karthikeyan M (2016) Therapeutic Effect of Amaranthus hybridus on Diabetic Nephropathy. J Develop Drugs 5:147.doi:10.4172/2329-6631.1000147 | |
SEE

Dr. T. Balasubramanian

Karthikeyan M
http://alshifacollegeofpharmacy.com/teaching-faculty.html








////////Therapeutic Effect, Amaranthus hybridus, Diabetic Nephropathy, AYURVEDA
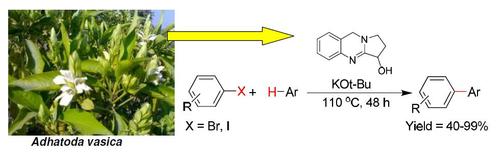
Vasicine (peganine) is a quinazoline alkaloid. It is the active compound of Justicia adhatoda, after which the chemical is named.
Vasicine has been compared to theophylline both in vitro and in vivo.[1] It has also been studied in combination with the related alkaloid vasicinone. Both the alkaloids in combination (1:1) showed pronounced bronchodilatory activity in vivo and in vitro.[2] Both alkaloids are also respiratory stimulants.[2] Vasicine has a cardiac–depressant effect, while vasicinone is a weak cardiac stimulant; the effect can be normalized by combining the alkaloids.[2][3] Vasicine is reported to have a uterine stimulant effect.[3]
Synonym Peganine
Biological Sources It is obtained from the leaves of Adhatoda vasica (L.) Nees (Acanthaceae) (Malabar Nut, Adotodai, Paveltia); and the seeds of Peganum harmala L. (Rutaceae) (Harmel, Syrian Rue, African Rue).
Chemical Structure
1, 2, 3, 9-Tetrahydropyrrolo [2, 1-b] quinazoline-3-ol; (C11H12N2O).
Isolation It is isolated from the leaves of Adhatoda vasica* and also from the seeds of Peganum harmala** by adopting the standard methods of isolation described earlier in this chapter.
Characteristic Features
dl-Form: 1. It is obtained as needles from ethanol having mp 210°C.
benzene.
l-Form: 1. It is obtained as needles from ethanol with mp 212°C.
Note: In dilute HCl it is obtained as its dextrorotatory form.
Identification Tests
Uses
Biosynthesis of Vasicine Various studies in Peganum harmala have evidently revealed vasicine (peganine) to be derived from the anthranilic acid, while the remaining portion of the structure comprising of a pyrrolidine ring provided by ornithine. The probable mechanism of vasicine skeleton may be explained by virtue of the nucleophilic attack from the N-atom present in anthranilate upon the pyrrolidinium cation, ultimately followed by amide formation. However, interestingly this pathway is not being adopted in Justicia adhatoda.

http://www.himalayawellness.com/products/pharmaceuticals/vasaka.htm
Vasaka (Malabar Nut Tree/Adhatoda zeylanica) is well known in Ayurveda for its beneficial effects in respiratory ailments, particularly as an expectorant in bronchitis. The leaves, flowers, fruits and roots are used extensively for treating cold, cough, whooping-cough, chronic bronchitis and asthma.
Vasaka grows throughout India, up to an altitude of 1,300 meters.
Vasaka contains the pyrroquinazoline alkaloids, including vasicine, vasicol and vasinone along with other minor constituents. Vasicine and vasinone are the major bioactive constituents of Vasaka which have bronchodilatory and antitussive properties.
The alkaloids present in the plant show significant protection against allergen-induced bronchial obstruction.
Respiratory care: Vasaka exhibits anti-inflammatory, antitussive and bronchodilatory action which eases congestion and coughing by helping loosen and thin mucus in airways. Vasaka relieves dyspnea by dilating the airways and improves overall lung functions. The herb is an excellent supportive therapy for symptomatic relief in tuberculosis and pulmonary infections.
None
One capsule, twice a day or as directed by your physician
Each capsule contains 250mg extract of Vasaka
Note: Since Himalaya’s Pure Herbs are in capsule form, some children below 14 years may find it difficult to swallow them. For this reason, Pure Herbs are recommended for children ages 14 and above.
The information on this page is not intended to be a substitute for professional medical advice. Do not use this information to diagnose or treat your problem without consulting your doctor.
http://kumarncsirihbt.weebly.com/publications.html

Adhatoda Vasica (Justicia Adhatoda) – Malabar Nut, Vasa, Vasaka …
Presentation “Herbal drugs for health Herbal drugs for health …
 |
|
| Names | |
|---|---|
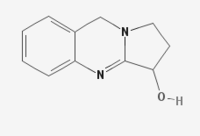
| IUPAC name
1,2,3,9-Tetrahydropyrrolo[2,1-b]quinazolin-3-ol
|
|
| Other names
Peganine
|
|
| Identifiers | |
| 6159-56-4 | |
| Jmol interactive 3D | Image |
| PubChem | 72610 |
| Properties | |
| C11H12N2O | |
| Molar mass | 188.23 g·mol−1 |
| Melting point | 210 °C (410 °F; 483 K) |
| Solubility in acetone, alcohol, chloroform | Soluble |
//////
Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process
Rachana Kamble, Sadhana Sathaye, DP Shah
University Institute of Chemical Technology, N. Parekh Marg, Matunga, Mumbai-400 019, India
According to ayurvedic texts shodhan vidhi is an important process which enhances the biological activity of a compound and reduces the toxicity at the same time. Before incorporating into formulations, guggul is processed using Shodhan vidhi involving different shodhan dravyas like gulvel, gomutra, triphala, dashmul. We have evaluated the antispasmodic activity of guggul on ileum of guinea pig and Wistar rats. The animals were sacrificed and ileum tissue of guinea pig and rat was isolated and tested for antispasmodic activity using different spasmogens like acetylcholine, histamine and barium chloride. It was observed that the different shodhit guggul (shudha guggul) i.e. processed using different shodhan vidhi, showed good antispasmodic activity as compared to Ashudha guggul. When acetylcholine was used as spasmogen, gulvel and triphala shodhit guggul showed good antispasmodic activity than other shodhit guggul. Thus shodhan vidhi enhances the therapeutic properties of guggul.
Kamble R, Sathaye S, Shah D P. Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process. Indian J Pharm Sci 2008;70:368-72
URL:
Kamble R, Sathaye S, Shah D P. Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process. Indian J Pharm Sci [serial online] 2008 [cited 2014 Dec 9];70:368-72. Available from: http://www.ijpsonline.com/text.asp?2008/70/3/368/43005


Associate professor of Pharmacy(Pharmacology) at Institute Of Chemical Technology

Zingerone Flower
Spice
In everyday life, ginger is used in cooking for its hot taste as well as its pungent smell.
|
Ecologists have studied the relationships of food and culture for many years. During this time, they have found surprising ties between spices that taste good and health-promoting side effects. An example of one of these spices is ginger. The sensory perception of ginger in the mouth and the nose arises from two distinct groups of chemicals:
|
|
|
|
Medicine
Ginger for many years has been the traditional remedy for colds. In modern medicine today, zingerone is used to treat a variety of medical problems. Zingerone reacts with free radicals that can cause tissue damage and inflammation. At Case Western University, research has been done showing that a topically applied extract containing zingerone may help prevent some skin cancers. In capsule form, ginger can also be used to replace anti-inflammatory drugs. In a recent study, ginger was found to be more effective than drugs in the treatment of nausea and motion sickness. Zingerone also has a major role in lipid oxidation since it is an anti-oxidant. It weakly inhibits oxidation of phospholipid liposomes in the presence of iron (III) and ascorbate to prevent heart-attacks.
 |
|
Zingerone used in pharmaceuticals |
It is these properties that have made zingerone a molecule of great importance and one that has been produced and synthesized for pharmaceutical use.
Structure
| 4-(4-hydroxy-3-methoxy-phenyl)-butan-2-one | 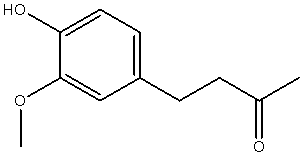 |
|
| Chemical Name | Structure of Zingerone | 3D Structure of Zingerone |
Physical Properties
| Synonyms | Zingerone; Vanillyl Acetone |
| Odour Description | Sweet, Spicy, Ginger, Vanilla, Woody |
| Appearance | Yellow To Yellow-Brown Crystals |
| Mol./Wt. | 194.2 |
| Formula | |
| Cas. # | 122-48-5 |
| Refractive Index | 1.54400 – 1.54500 @ 20.00 �C. |
| Melting Point | 40 – 41�C (Solvent – Aq. Ethanol) |
| Boiling Point | 141 �C. @ 0.5 Torr |
| Boiling Point | 290�C. @ 760.00 mmHg |
| Soluble in | Ethyl Alcohol, 1:1 In 50% Alcohol |
| Natural Occurrence | Ginger Root; Raspberry; Zingiber Officinale |
Mass Spectrum of Zingerone
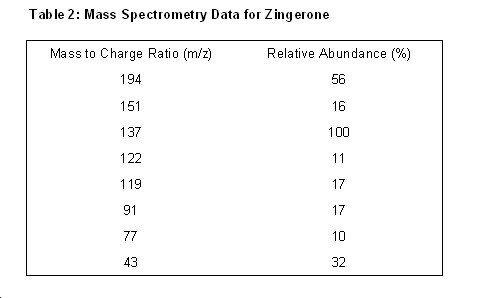
Pattern for Mass Fragmentation in the Spectrum
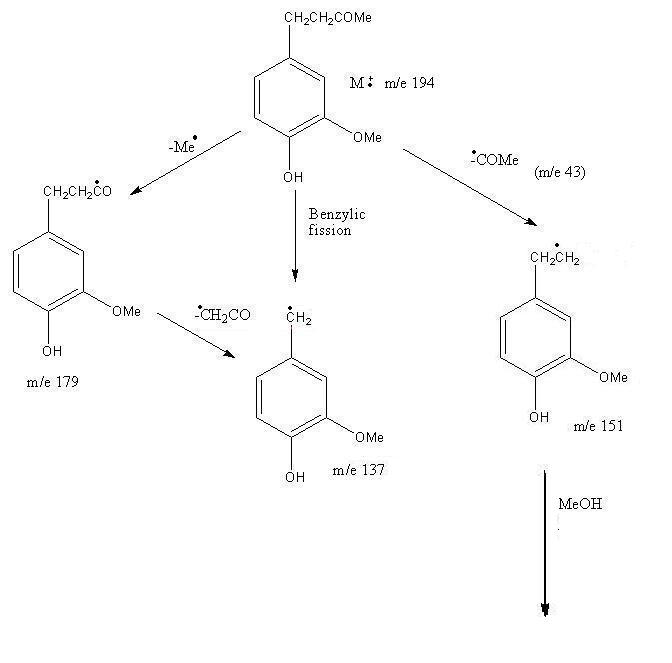
Infrared Spectrum of Zingerone
Table 3 : Infra Red spectral data on Zingerone

NOMURA METHOD
The Bunce & Reeves Method
Historical
Zingerone has been extracted from ginger for the past two thousand years.
A Brief History
| Common Name: | Ginger |
||||||||
| Latin Name: | Zingiber Officinale | ||||||||
| Family: | zingiberaceae | ||||||||
| Other Names: | Based on its origin:
|
The word ginger comes from the ancient Sanskrit ‘singabera’, meaning ‘shaped like a horn’. It first appeared in the writings of Confucius in the 5th century BC. and it has been used medicinally in the West for the past 2000 years. Various virtues have been ascribed to the spice; e.g. Henry VIII recommended it as a pro-phylactic against the plague. It was introduced by the Spaniards to the Americas and is now cultivated extensively in the West Indies. The Portuguese introduced it to West Africa. It is now used all over the world.
Only in the past century has zingerone been produced synthetically. A few interesting and unique syntheses have been chosen. Down the years, the technique of synthesis evolved. Though Lapworth & Wykes were the pioneers in the synthesis of zingerone, their method and reagents were not repeated. Below is a diagram of the apparatus used in their experiment.
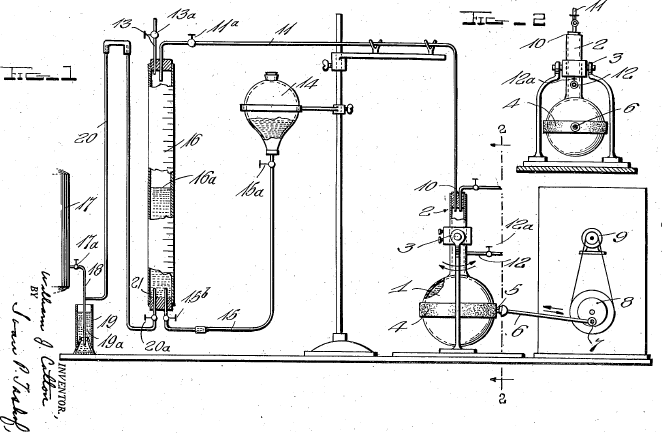
Nomura, then Mannich and Merz founded the method used today. This is shown by Kim and Kim’s work. The other interesting method was formed by Bunce and Reeves. This method and the common one used today are elaborated on in the preparation section.
|
Year |
Method | Reagents |
| 1917 | Reduction and Decarboxylation of ethyl vanillylideneacetoacetateLapworth & Wykes | The Pioneers in the synthesis.Na Amalgam and NaOH solution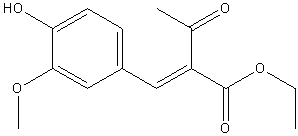
|
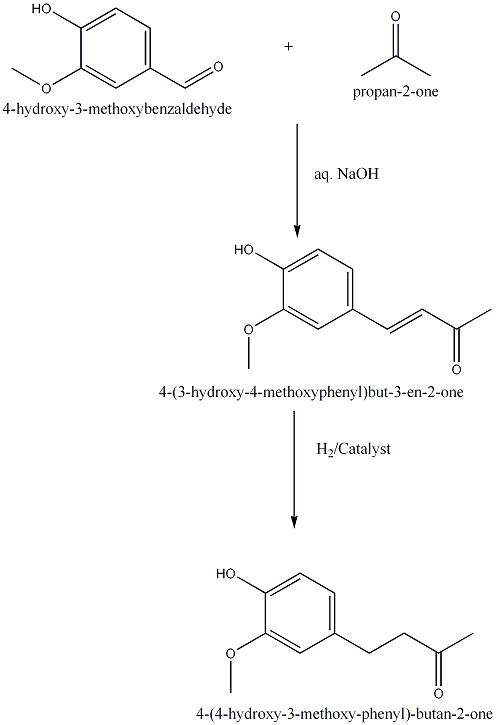
| 1925 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Nomura | Na Amalgam and Water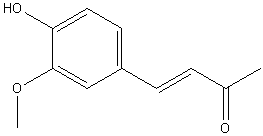 |
| 1927 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Mannich & Merz | Pd Catalyst, |
| 1979 | Reaction of 4-benzyloxy-3-methoxybromomethylbenzene with the anion of acetone dimethylhydrazone, followed by oxidative hydrolysis, then hydrogenolysis to remove the benzyl groupEnders D. et Al | Pd/C, Hydrogen, Methanol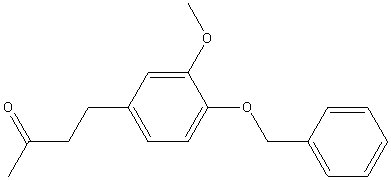 |
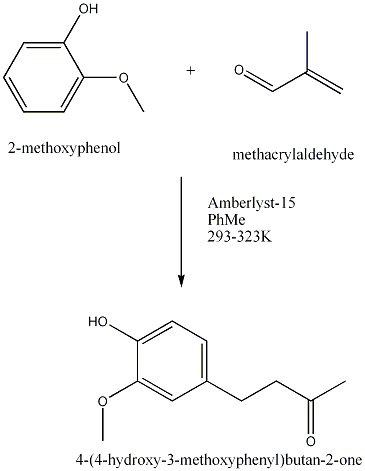
| 1989 | Amberlyst-15-catalyzed addition of 3-buten-2-one to the phenolBunce & Reeves | Amberlyst-15, Toluene, 20-50�C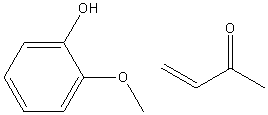 |
| 2004 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Kim D. & Kim J. Y. | Pd/C Catalyst, |
Spice
 |
| Ginger |
As commented upon in the introduction, zingerone puts the ‘zing’ in ginger. In mustard oil, zingerone uses its properties to give it its flavour.
Zingerone has properties that give its strength of flavour. The higher molecular weight of zingerone in combination with the polar side-chain carbonyl group make zingerone molecules attract each other strongly. As a result, zingerone is not that volatile. The odour of ginger isn’t strong, but the hydrocarbon tail gives it a more intense flavour when it does come into contact with its receptor. Zingerone is also used in artificial flavouring and in fragrances.
A company called “Aroma Fragrance Fine Chemicals” (AFFC) have formulations are used globally for imparting attractive taste and aroma to processed foods and beverages. These chemicals can also add pleasing scents to perfumes, toiletries and detergents. Zingerone is one of their formulations. It is both a flavour and fragrance ingredient. This therefore means that zingerone is important in both processed foods and perfumes.
In industry, zingerone is therefore synthesised using the Kim & Kim’s method for this purpose.
Zingerone contains vanilloid (3-methoxy-4-hydroxy benzene) group in its structure. These phenolic hydroxyl groups provide the possibility to introduce a 4-ether-linked propanolamine side chain. Propanolamine derivatives were obtained by reacting zingerone with epichlorohydrin, and the obtained epoxide compounds were then reacted with isopropylamine, tert-butylamine or guaiacoxyethylamine, respectively, to yield 3 new derivatives shown below.
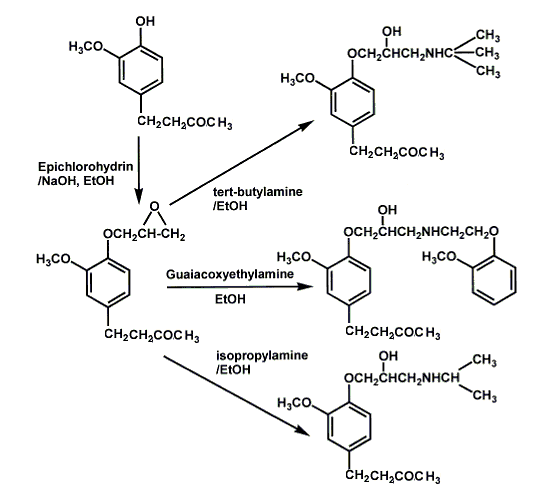
The syntheses above create third-generation β-adrenoceptor blockers, which possess ancillary cardiovascular actions other than β-adrenoceptor blockade, or cardioselective β-adrenoceptor blockers, have been shown to improve left ventricular function and decrease the risk of chronic heart failure. These β-adrenoceptor blockers are a break through in science and make the synthesis of zingerone very important in industry.

Aesculus
 |
|
| Aesculus hippocastanum |
The genus Aesculus (/ˈɛskjʊləs/[1] or /ˈaɪskjʊləs/) comprises 13–19 species of trees and shrubs native to the temperate Northern Hemisphere, with 6 species native to North America and 7–13 species native to Eurasia; there are also several hybrids. Aesculus exhibits a classical arcto-Tertiary distribution.[a] The genus has traditionally been treated in the ditypic family Hippocastanaceae along with Billia,[3] but recent phylogenetic analysis of morphological[4] and molecular data[5] has caused this family, along with the Aceraceae (Maples andDipteronia), to be included in the soapberry family (Sapindaceae).
Linnaeus named the genus Aesculus after the Roman name for an edible acorn. Common names for these trees include “buckeye” and “horse chestnut”. Some are also called white chestnut or red chestnut (as in some of the Bach flower remedies). In Britain, they are sometimes called conker trees because of their link with the game of conkers, played with the seeds, also called conkers. Aesculus seeds were traditionally eaten, after leaching, by the Jōmon people of Japan over about four millennia, until 300 AD.[6]

Aesculus glabra Ohio buckeye

Flower of Aesculus x carnea, the red Horse Chestnut
Aesculus species have stout shoots with resinous, often sticky, buds; opposite, palmately divided leaves, often very large—to 65 cm (26 in) across in the Japanese horse chestnut Aesculus turbinata. The seeds of the Aesculus are traditionally used in a game called conkers in Europe. Species are deciduous or evergreen. Flowers are showy, insect- or bird-pollinated, with four or five petals fused into a lobedcorolla tube, arranged in a panicle inflorescence. Flowering starts after 80–110 growing degree days. The fruit matures to a capsule, 2–5 cm (25⁄32–1 31⁄32 in) diameter, usually globose, containing one to three seeds (often erroneously called a nut) per capsule. Capsules containing more than one seed result in flatness on one side of the seeds. The point of attachment of the seed in the capsule (hilum) shows as a large circular whitish scar. The capsule epidermis has “spines” (botanically: prickles) in some species, while other capsules are warty or smooth. At maturity, the capsule splits into three sections to release the seeds.[7][8][9]
The species of Aesculus include:
The most familiar member of the genus worldwide is the common horse chestnut Aesculus hippocastanum. The yellow buckeye Aesculus flava (syn. A. octandra) is also a valuable ornamental tree with yellow flowers, but is less widely planted. Among the smaller species, the bottlebrush buckeye Aesculus parviflora also makes a very interesting and unusual flowering shrub. Several other members of the genus are used as ornamentals, and several horticultural hybrids have also been developed, most notably the red horse chestnut Aesculus × carnea, a hybrid between A. hippocastanum and A. pavia.
Aesculus has been listed as one of the 38 substances used to prepare Bach flower remedies,[10] a kind of alternative medicine promoted for its effect on health. However according to Cancer Research UK, “there is no scientific evidence to prove that flower remedies can control, cure or prevent any type of disease, including cancer”.[11]
Aesculus hippocastanum is a large deciduous tree, commonly known as horse-chestnut or conker tree.
Aesculus hippocastanum is native to a small area in the Pindus Mountains mixed forests and Balkan mixed forests of South East Europe.[1]It is widely cultivated in streets and parks throughout the temperate world.
A. hippocastanum grows to 36 metres (118 ft) tall, with a domed crown of stout branches; on old trees the outer branches often pendulous with curled-up tips. The leaves are opposite and palmately compound, with 5–7 leaflets; each leaflet is 13–30 cm long, making the whole leaf up to 60 cm across, with a 7–20 cm petiole. The leaf scars left on twigs after the leaves have fallen have a distinctive horseshoe shape, complete with seven “nails”. The flowers are usually white with a small red spot; they are produced in spring in erect panicles 10–30 cm tall with about 20–50 flowers on each panicle. Usually only 1–5 fruit develop on each panicle; the shell is a green, spiky capsule containing one (rarely two or three) nut-like seeds called conkers or horse-chestnuts. Each conker is 2–4 cm diameter, glossy nut-brown with a whitish scar at the base.[2]
The common name “horse-chestnut” (often unhyphenated) is reported as having originated from the erroneous belief that the tree was a kind of chestnut (though in fact only distantly related), together with the observation that eating the fruit cured horses of chest complaints[3] despite this plant being poisonous to horses.
Cultivation for its spectacular spring flowers is successful in a wide range of temperate climatic conditions provided summers are not too hot, with trees being grown as far north asEdmonton, Alberta, Canada,[4] the Faroe Islands,[5] Reykjavík, Iceland and Harstad, Norway.
In Britain and Ireland, the nuts are used for the popular children’s game conkers. During the First World War, there was a campaign to ask for everyone (including children) to collect horse-chestnuts and donate them to the government. The conkers were used as a source of starch for the fermentation via the Clostridium acetobutylicum method devised by Chaim Weizmann to produce acetone. Any starch plant would have done, but they chose to ask for conkers to avoid causing starvation by using food. Weizmann’s process could use any source of starch, but it was never particularly efficient and the factory only produced acetone for three months. The aim was to produce acetone for use as solvent which aided in the production of cordite, which was then used in military armaments.
The nuts, especially those that are young and fresh, are slightly poisonous, containing alkaloid saponins and glucosides. Although not dangerous to touch, they cause sickness when eaten; consumed by horses, they can cause tremors and lack of coordination.[6] Somemammals, notably deer, are able to break down the toxins and eat them safely.[citation needed]
Though the seeds are said to repel spiders there is little evidence to support these claims. The presence of saponin may repel insects but it is not clear whether this is effective on spiders.[7]
Horse-chestnuts have been threatened by the leaf-mining moth Cameraria ohridella, whose larvae feed on horse chestnut leaves. The moth was described from Macedonia where the species was discovered in 1984 but took 18 years to reach Britain.[8]
The flower is the symbol of the city of Kiev, capital of Ukraine.[9] Although the horse-chestnut is sometimes known as the buckeye, this name is generally reserved for the New World members of the Aesculus genus.
The seed extract standardized to around 20 percent aescin (escin) is used for its venotonic effect, vascular protection, anti-inflammatory and free radical scavenging properties.[10][11] Primary indication is chronic venous insufficiency.[11][12] A recent Cochrane Review found the evidence suggests that Horse Chestnut Seed Extract is an efficacious and safe short-term treatment for chronic venous insufficiency.[13]
Aescin reduces fluid leaks to surrounding tissue by reducing both the number and size of membrane pores in the veins.
Two preparations are considered; whole horsechestnut extract (whole HCE) and purified β-aescin. Historically, whole HCE has been used both for oral and IV routes (as of year 2001). The rate of adverse effects are low, in a large German study, 0.6%, consisting mainly of gastrointestinal symptoms. Dizziness, headache and itching have been reported. One serious safety issue is rare cases of acute anaphylactic reactions, presumably in a context of whole HCE. Purified β-aescin would be expected to have a better safety profile.
Another is the risk of acute renal failure, “when patients, who had undergone cardiac surgery were given high doses of horse chestnut extract i.v. for postoperative oedema. The phenomenon was dose dependent as no alteration in renal function was recorded with 340 μg kg−1, mild renal function impairment developed with 360 μg kg−1 and acute renal failure with 510 μg kg−1”.[14] This almost certainly took place in a context of whole HCE.
Three clinical trials were since performed to assess the effects of aescin on renal function. A total of 83 subjects were studied; 18 healthy volunteers given 10 or 20 mg iv. for 6 days, 40 in-patients with normal renal function given 10 mg iv. two times per day (except two children given 0.2 mg/kg), 12 patients with cerebral oedema and normal renal function given a massive iv. dose on the day of surgery (49.2 ± 19.3 mg) and 15.4 ± 9.4 mg daily for the following 10 days and 13 patients with impaired renal function due to glomerulonephritis or pyelonephritis, who were given 20–25 mg iv. daily for 6 days. “In all studies renal function was monitored daily resorting to the usual tests of renal function: BUN, serum creatinine, creatinine clearance, urinalysis. In a selected number of cases paraaminohippurate and labelled EDTA clearance were also measured. No signs of development of renal impairment in the patients with normal renal function or of worsening of renal function in the patients with renal impairment were recorded.” It is concluded that aescin has excellent tolerability in a clinical setting.[15]
Raw Horse Chestnut seed, leaf, bark and flower are toxic due to the presence of esculin and should not be ingested. Horse chestnut seed is classified by the FDA as an unsafe herb.[11] The glycoside and saponin constituents are considered toxic.[11]
Aesculus hippocastanum is used in Bach flower remedies. When the buds are used it is referred to as “chestnut bud” and when the flowers are used it is referred to as “white chestnut”.
Quercetin 3,4′-diglucoside, a flavonol glycoside can also be found in horse chestnut seeds.[16] Leucocyanidin, leucodelphinidin and procyanidin A2 can also be found in horse chestnut.
A famous specimen of the horse-chestnut was the Anne Frank Tree in the centre of Amsterdam, which she mentioned in her diary and which survived until August 2010, when a heavy wind blew it over.[17][18] Eleven young specimens, sprouted from seeds from this tree, were transported to the United States. After a long quarantine in Indianapolis, each tree was shipped off to a new home at a notable museum or institution in the United States, such as the 9/11 Memorial Park, Central H.S. in Little Rock, and two Holocaust Centers. One of them was planted outdoors in March 2013 in front of the Children’s Museum of Indianapolis, where they were originally quarantined. [1]
The horse-chestnut is a favourite subject for bonsai.[19]
| Name | Language | First published | Last updated |
|---|---|---|---|
| Final Community herbal monograph on Aesculus hippocastanum L., cortex | (English only) | 27/06/2012 | |
| Opinion of theHMPC on a Community herbal monograph on Aesculus hippocastanum L., cortex | (English only) | 27/06/2012 | |
| Final assessment report on Aesculus hippocastanum L., cortex | (English only) | 27/06/2012 | |
| Final list of references supporting the assessment of Aesculus hippocastanum L., cortex | (English only) | 27/06/2012 | |
| Overview of comments received onCommunity herbal monograph on Aesculus hippocastanum L., cortex |
| Draft Community herbal monograph on Aesculus hippocastanum L., cortex | (English only) | 26/10/2011 | |
| Draft assessment report on Aesculus hippocastanum L., cortex | (English only) | 26/10/2011 | |
| Draft list of references supporting the assessment of Aesculus hippocastanum L., cortex | (English only) | 26/10/2011 | |
| Procedure for calls for scientific data for use in HMPCassessment work |
| Latin name of the genus | Aesculus |
|---|---|
| Latin name of herbal substance | Hippocastani cortex |
| Botanical name of plant | Aesculus hippocastanum L. |
| English common name of herbal substance | Horse-chestnut bark |
| Status | F: Assessment finalised |
| Date added to the inventory | 06/05/2010 |
| Date added to priority list | 06/05/2010 |
| Outcome of European assessment | Community herbal monograph |
| Latin name of the genus | Aesculus |
|---|---|
| Latin name of herbal substance | Hippocastani semen |
| Botanical name of plant | Aesculus hippocastanum L. |
| English common name of herbal substance | Horse-Chestnut Seed |
| Status | F: Assessment finalised |
| Date added to the inventory | 07/09/2006 |
| Date added to priority list | 07/09/2006 |
| Outcome of European assessment | Community herbal monograph |
| Name | Language | First published | Last updated |
|---|---|---|---|
| Final community herbal monograph on Aesculus hippocastanum L., semen | (English only) | 16/07/2009 | |
| Opinion of the Committee on Herbal Medicinal products on a community herbal monograph on Aesculus Hippocastanum L., semen | (English only) | 16/07/2009 | |
| Final list of references for assessment of: Hippocastani semen Aesculus hippocastanum L., semen (horse chestnut seed) | (English only) | 16/07/2009 | |
| Assessment report on Aesculus hippocastanum L., semen | (English only) | 16/07/2009 | |
| Overview of comments received on community herbal monograph on Aesculus hippocastanum L., semen (EMEA/HMPC/225319/2008) | (E |

Rosemary is a wonderful herb with a tradition of use spanning millennia. It has innumerable uses in both the kitchen and in herbal medicine.
read at
http://www.herbs-info.com/blog/scientists-find-sniffing-rosemary-can-increase-memory-by-75/

 artemisinin
artemisininOther common name(s): absinthium, absinth wormwood
Scientific/medical name(s): Artemisia absinthium
Wormwood is a shrubby perennial plant whose upper shoots, flowers, and leaves are used in herbal remedies and as a bitter flavoring for alcoholic drinks. It is native to Europe, northern Africa, and western Asia, and now also grows in North America.
Available scientific evidence does not support claims that wormwood is effective in treating cancer, the side effects of cancer treatment, or any other conditions. The plant contains a volatile oil with a high level of thujone (see Thuja). There are reports that taking large doses of wormwood internally can cause serious problems with the liver and kidneys. It can also cause nausea, vomiting, stomach pain, headache, dizziness, seizures, numbness of the legs and arms, delirium, and paralysis.
Wormwood, or Artemisia absinthium, should not be confused with sweet wormwood, or Artemisia annua. Although wormwood is related to sweet wormwood, they are used in different ways. Extracts of sweet wormwood have been used in traditional herbal medicine, and an active ingredient, artemisinin, is now used in conventional medical treatment of malaria.
Wormwood is promoted as a sedative and anti-inflammatory. There are also claims that it can treat loss of appetite, stomach disorders, and liver and gallbladder complaints. In folk medicine it is used for a wide range of stomach disorders, fever, and irregular menstruation. It is also used to fight intestinal worms. Externally, it is applied to poorly healing wounds, ulcers, skin blotches, and insect bites. It is used in Moxibustion treatments for cancer (seeMoxibustion). Available scientific evidence does not support these claims.
Wormwood is taken in small doses for a short period of time, usually a maximum of 4 weeks. It is available as a capsule and as a liquid that can be added to water to make a tincture. The whole herb is sometimes brewed as a tea. Wormwood oil, washes, or poultices can also be used on the skin. Although pure wormwood is not available, “thujone-free” wormwood extract has been approved by the US Food and Drug Administration (FDA) for use in foods and as a flavoring in alcoholic drinks such as vermouth.
Artemisia absinthium was used by Hippocrates, and the earliest references to wormwood in Western civilization can be found in the Bible. Extract of wormwood was also used in ancient Egypt. The herb is mentioned often in first-century Greek and Roman writings and reportedly was placed in the sandals of Roman soldiers to help soothe their sore feet. It was taken as a treatment for tapeworms as far back as the Middle Ages.
In 1797, Henri Pernod developed absinthe, an alcoholic drink containing distilled spirits of wormwood, fennel, anise and sometimes other herbs. Absinthe became very popular in Europe and the United States in the nineteenth century. It was eventually banned in several countries in the early twentieth century due to its purported ill effects and addictive qualities. More recent analysis has suggested that, when properly prepared and distilled, the thujone content in these drinks was very low. It appears more likely that the addictiveness and other ill effects of absinthe were due to its alcohol content, which is around 60% to 85%. Varying additives or impurities from different distillers may have also produced some of these effects. Even though absinthe is illegal in some countries, various types can be found in some European countries. However, their thujone content is strictly limited. Wormwood is also an ingredient in vermouth and other drinks.
Available scientific studies do not support the use of wormwood for the treatment of cancer or the side effects of conventional cancer treatment. There is not enough evidence available to support its use for other conditions. Wormwood oil has been tested in laboratory studies and appears to inhibit the growth of some fungi. However, human tests have not been completed.
Some derivatives of Artemisia annua, or sweet wormwood, a relative of wormwood, have been shown to be effective in the treatment of malaria. In fact, the World Health Organization approved artemisinin for use against malaria in Africa in 2004. These extracts also show some promise in laboratory studies as cancer treatment drugs. Further studies are required to find out whether the anti-cancer results apply to people. It is important to remember that extracted compounds are not the same as the whole herb, and study results are not likely to show the same effects.
Wormwood should be avoided, especially by women who are pregnant or breast-feeding, by people who have had seizures, and by those with ulcers or stomach irritation. Thujone, a component of wormwood, is known to cause muscle spasms, seizures, and hallucinations if taken internally. In high doses it is known to damage the liver and the kidneys.
Because of its thujone content, large doses of wormwood taken internally can lead to vomiting, stomach and intestinal cramps, headaches, dizziness, nervous system problems, and seizures. Wormwood can also lead to liver failure. The New England Journal of Medicine reported that a man who ordered essential oil of wormwood over the Internet, thinking he had purchased absinthe, suffered liver failure shortly after drinking the oil. Wormwood may also make seizures more likely and may interfere with the anti-convulsant effects of medicines such as phenobarbital.
The plant is a relative of ragweed and daisies. Those with allergies to these types of plants may also be allergic to wormwood. Contact with wormwood can cause rash in some people.
Relying on this type of treatment alone and avoiding or delaying conventional medical care for cancer may have serious health consequences.
Vitamin D is an essential vitamin required by the body for the proper absorption of calcium, bone development, control of cell growth, neuromuscular functioning, proper immune functioning, and alleviation of inflammation. A deficiency in vitamin D can lead to rickets, a disease in which bones fail to properly develop. Further, inadequate levels of vitamin D can lead to a weakened immune system, increased cancer risk, poor hair growth, and osteomalacia, a condition of weakened muscles and bones. Conversely, excess vitamin D can cause the body to absorb too much calcium, leading to increased risk of heart attack and kidney stones. The current U.S. DV for vitamin D is 600 IU (international units) and the toxicity threshold for vitamin D is thought to be 10,000 to 40,000 IU/day.2 Vitamin D is oil soluble, which means you need to eat fat to absorb it. It is naturally found mainly in fish oils, fatty fish, and to a lesser extent in beef liver, cheese, egg yolks, and certain mushrooms. Vitamin D is also naturally made by your body when you expose your skin to the sun, and thus, is called the sun-shine vitamin. In addition, vitamin D is widely added to many foods such as milk and orange juice, and can also simply be consumed as a supplement. Below is a list of high vitamin D foods.
1: Cod Liver Oil
 Cod liver oil has been a popular supplement for many years and naturally contains very high levels of vitamin A and vitamin D. Cod liver oil provides 10001IU (1667% DV) per 100 gram serving, or 1360IU (340% DV) in a single tablespoon.
Cod liver oil has been a popular supplement for many years and naturally contains very high levels of vitamin A and vitamin D. Cod liver oil provides 10001IU (1667% DV) per 100 gram serving, or 1360IU (340% DV) in a single tablespoon.
2: Fish
 Various types of fish are high in vitamin D. Typically raw fish contains more vitamin D than cooked, and fatty cuts will contain more than lean cuts. Further, fish canned in oil will have more vitamin D than those canned in water. Raw fish is typically eaten in the form of sushi. Raw Atlantic Herring provides the most vitamin D with 1628IU (271% DV) per 100 gram serving, 2996IU (499% DV) per fillet, and 456IU (76% DV) per ounce. It is followed by Pickled Herring with 680IU (113% DV) per 100g serving, Canned Salmon (127% DV), Raw Mackerel (60% DV), Oil Packed Sardines (45% DV), Canned Mackerel (42% DV), and oil packed Tuna (39% DV).
Various types of fish are high in vitamin D. Typically raw fish contains more vitamin D than cooked, and fatty cuts will contain more than lean cuts. Further, fish canned in oil will have more vitamin D than those canned in water. Raw fish is typically eaten in the form of sushi. Raw Atlantic Herring provides the most vitamin D with 1628IU (271% DV) per 100 gram serving, 2996IU (499% DV) per fillet, and 456IU (76% DV) per ounce. It is followed by Pickled Herring with 680IU (113% DV) per 100g serving, Canned Salmon (127% DV), Raw Mackerel (60% DV), Oil Packed Sardines (45% DV), Canned Mackerel (42% DV), and oil packed Tuna (39% DV).
3: Fortified Cereals

4: Oysters
 In addition to vitamin D, Oysters are a great source of vitamin b12, zinc, iron, manganese, selenium, and copper. Oysters are also high in cholesterol and should be eaten in moderation by people at risk of heart disease or stroke. Raw wild caught Eastern Oysters provide 320IU (80% DV) per 100 gram serving, 269IU (67% DV) in six medium oysters.
In addition to vitamin D, Oysters are a great source of vitamin b12, zinc, iron, manganese, selenium, and copper. Oysters are also high in cholesterol and should be eaten in moderation by people at risk of heart disease or stroke. Raw wild caught Eastern Oysters provide 320IU (80% DV) per 100 gram serving, 269IU (67% DV) in six medium oysters.
5: Caviar (Black and Red)
 Caviar is a common ingredient in sushi and more affordable than people think. Caviar provides 232IU (58% DV) of vitamin D per 100 gram serving, or 37.1IU (9% DV) per teaspoon.
Caviar is a common ingredient in sushi and more affordable than people think. Caviar provides 232IU (58% DV) of vitamin D per 100 gram serving, or 37.1IU (9% DV) per teaspoon.
6: Fortified Soy Products (Tofu and Soy Milk)
 Fortified soy products are often fortified with both vitamin D and calcium. Fortified Tofu can provide up to 157IU (39% DV) of vitamin D per 100 gram serving, or 44IU (11% DV) per ounce. Fortified Soy Milk can provide up to 49IU (12% DV) of vitamin D per 100 gram serving, 119IU (30% DV) per cup. Amounts of vitamin D vary widely between products, so be sure to check nutrition facts for vitamin D content.
Fortified soy products are often fortified with both vitamin D and calcium. Fortified Tofu can provide up to 157IU (39% DV) of vitamin D per 100 gram serving, or 44IU (11% DV) per ounce. Fortified Soy Milk can provide up to 49IU (12% DV) of vitamin D per 100 gram serving, 119IU (30% DV) per cup. Amounts of vitamin D vary widely between products, so be sure to check nutrition facts for vitamin D content.
7: Salami, Ham, and Sausages
 Salami, Ham, and Sausages are a good source of vitamin b12, and copper. Unfortunately, they are also high in cholesterol and sodium, and so should be limited by people at risk of hypertension, heart attack, and stroke. Salami provides 62.0IU (16% DV) of vitamin D per 100 gram serving, or 16.7IU (4% DV) per ounce (3 slices). It is followed by Bologna Pork 56IU (9% DV) per 100 grams, and Bratwurst 44IU (7% DV) per 100 gram serving.
Salami, Ham, and Sausages are a good source of vitamin b12, and copper. Unfortunately, they are also high in cholesterol and sodium, and so should be limited by people at risk of hypertension, heart attack, and stroke. Salami provides 62.0IU (16% DV) of vitamin D per 100 gram serving, or 16.7IU (4% DV) per ounce (3 slices). It is followed by Bologna Pork 56IU (9% DV) per 100 grams, and Bratwurst 44IU (7% DV) per 100 gram serving.
8: Fortified Dairy Products
 Dairy products are already high in calcium, so it makes sense to fortify them with vitamin D. Milk can provide up to 52.0IU (13% DV) of vitamin D per 100 gram serving, 127IU (32% DV) per cup. Cheese can provide up to 6.6IU (2% DV) in a cubic inch, and butter provides 7.8IU (2% DV) in a single tablespoon. Check nutrition labels for exact amounts.
Dairy products are already high in calcium, so it makes sense to fortify them with vitamin D. Milk can provide up to 52.0IU (13% DV) of vitamin D per 100 gram serving, 127IU (32% DV) per cup. Cheese can provide up to 6.6IU (2% DV) in a cubic inch, and butter provides 7.8IU (2% DV) in a single tablespoon. Check nutrition labels for exact amounts.
9: Eggs
 In addition to vitamin D, eggs are a good source of vitamin B12, and protein. Eggs provide 37.0IU (9% DV) of vitamin D per 100 gram serving, or 17.0IU (4% DV) in a large fried egg.
In addition to vitamin D, eggs are a good source of vitamin B12, and protein. Eggs provide 37.0IU (9% DV) of vitamin D per 100 gram serving, or 17.0IU (4% DV) in a large fried egg.
10: Mushrooms
 More than just a high vitamin D food, mushrooms also provide Vitamin B5 (Pantothenic Acid) and copper. Lightly cooked white button mushrooms provide the most vitamin D with 27.0IU (7% DV) per 100 gram serving, or 7.6IU (2% DV) per ounce.
More than just a high vitamin D food, mushrooms also provide Vitamin B5 (Pantothenic Acid) and copper. Lightly cooked white button mushrooms provide the most vitamin D with 27.0IU (7% DV) per 100 gram serving, or 7.6IU (2% DV) per ounce.
(NaturalNews) Various cultures around the world already understand that herbs such as ginger, cinnamon, garlic and turmeric can effectively treat conditions like diabetes. According to research from the biomedical science department at the King Faisal University in Saudi Arabia, garlic may be the most powerful of them all for treating diabetes.
Learn more: http://www.naturalnews.com/042941_garlic_diabetes_treatment_oxidative_stress.html##ixzz2l5NBhJ00