BIOSIMILARS

Introduction to Biosimilars
A Biosimilar is a biological medicine that is similar to, but not identical to, another biological medicine that has already been approved
Due to its complex molecular structure and unique manufacturing process, a Biosimilar is not a copy of its originator biologics
Concept of a “similar biological medicinal product” was adopted in EU pharmaceutical legislation in 2004
EU is the first region in the world to have set up a legal framework and a regulatory pathway for Biosimilars
EU regulatory framework inspired many countries(Australia, Canada, Japan, USA)
Coined by EMA (European Medicine Agency)
Bio-betters
Similar Biological Products (SBP)
Follow-on bioproducts
“Generics of Bio-pharmaceutical Industry”
Caution : Not alternative biologics
What are biosimilars?
- Legally approved subsequent versions of innovator biopharmaceutical products made by a different sponsor following patent & exclusivity expiry of the innovator product.
- Because of structural & manufacturing complexities, these biological products are considered as similar, but not generic equivalents of innovator biopharmaceuticals
DEFINITION OF BIOSIMILARS
SBP (Similar Biologic Product)
A biotherapeutic product which is similar in terms of quality, safety and efficacy to an already licensed reference biotherapeutic product
FOB (Follow-On Biologic)
A biological product that is highly similar to a U.S. licensed reference biological product with no clinically meaningful differences in terms of the safety, purity and potency .
Based on these different definitions, Three determinants in definition of biosimilar product:
- It should be a biologic product
- Reference product should be an already licensed biologic product
- Demonstration of high similarity in safety, quality & efficacy is necessary Ø Similarity should be demonstrated using a set of comprehensive comparability exercises at the quality, non-clinical & clinical level
- Biosimilars Unlike generic medicines where the active ingredients are identical, biosimilars – by definition are not likely to be identical to the originator biologic. Similar to Snowflakes Biologics made by different manufacturers differ from the original product and from each other
Biosimilars As A Healthcare Concept Layers of “The Concept” –
- Scientific Layer
- Concept of similarity in term of bio-characterization, potency, safety & effect w.r.t innovator molecule • Predictable PK & PD based on data for reference compound
- Regulatory Layer
- Faster & Easier approval route
- Early market entry,
- Reduced Clinical trial requirements (extrapolation on basis of data available for the innovator molecule )
- Market and Social Layer
- Established & Larger Market Base and distribution channels
- Cost- Demand trade off,
- Increased Profit Margins for sponsors
- Affordability
Lets come back to India
- Emerging Biopharma hub , Robust Manufacturing Base
- Itself a big market, semi-regulated (Easier market entry + High demand = PROFITS)
- Regulation Central Drug Standard Control Organization CDSCO & DBT recently drafted Pre-market Regulatory Compliances : Guidelines on Similar Biologics (2012)
Indian Players in Biosimilars
- Trends • Target Markets : Domestic + Developed Market ( as Contract Manufacturers)
- Stronger Acquisition Strategy : Capacity Building for production
- Aiming for Regulated Markets like US and EU also
- Strong Response to Tap Opportunity from: Biocon, Dr. Reddy’s Lab., Wockhardt, Cipla Pharmaceuticals
Hurdles
- Proper Regulatory structure not in place yet.
- A competitive edge to original manufacturer (good knowledge base for manufacturing a Biosimilars based on innovator biologic production)
- Proof of Similarity and comparability to innovator product requires very hectic characterization. (LOT OF ANALYSIS COMPARED TO GENERICS ) A lot of Analytic Techniques required for validation ( IEX (charge), RPLC, SEC, AXC , CE etc )
What can be done ?
- Post Approval Risk Plan – Strong communication between front end of healthcare system (Doctors) to the manufacturer and regulatory authorities. – Continuation of post approval trials
- Option between a well established (but costly) biologics and A newly introduced Biosimilars should be left to patient’s will.
- Streamlined regulatory pathways for global platform
Production of biologic
The genetic code of a chosen protein, such as immune system antibody is identified and replicated by combining different segments of DNA to build a functional DNA sequence
This DNA sequence is introduced into the host cell of a living organism, such as mammal cells altering the cell to produce the chosen protein.
These genetically modified cell lines are carefully selected (MASTER CELL LINE) and cultured in large bioreactors before the biologic medicine is extracted through complex and lengthy purification process
Differences between chemical generics & biosimilars
- Heavier Unlike structurally well-defined, low molecular weight chemical drugs, biopharmaceuticals are: Ø High molecular weight compounds with complex three-dimensional structure Ø For example, the molecular weight of Aspirin is 180 Da whereas Interferon-β is 19,000 Da
- Larger ØTypical biologic drug is 100 to 1000 times larger than small molecule chemical drugs. Ø Possesses fragile threedimensional structure as compared to wellcharacterized one-dimensional structure of chemical drug.
- Difficult to define structure ØSmall Molecule drugs → easy to reproduce & specify by mass spectroscopy & other techniques. Ø Lack of appropriate investigative tools to define composite structure of large proteins
- Complex manufacturing processes Ø Manufacturers of biosimilar products will not have access to manufacturing process of innovator products→ Proprietary knowledge Ø Impossible to accurately duplicate any protein product Ø Different manufacturing processes use different cell lines, protein sources & extraction & purification techniques→ heterogeneity of biopharmaceuticals
Versatile cell lines used to produce the proteins have an impact on the gross structure of the protein
Such alterations may significantly impact: Receptor binding, Stability, Pharmacokinetics & Safety
Immunogenic potential of therapeutic proteins→ Unique safety issue→ Not observed with chemical generics
Benefits of Biosimilars
“The development of biosimilars allows for wider and, as important, earlier access to these agents because of their lower cost and consequently greater affordability” “lower cost is expected not only to improve costefficacy ratios, but also to improve drug access, “
Emerging Role of Biosimilars Ø
Countries around the world- growing, aging population —> ↑ in chronic disease. Ø Expanding demand for good-quality healthcare —> challenge of controlling healthcare expenditure. Ø The safe and regulated introduction of biosimilars into the market has been forecasted to increase access to much needed biologic medicines and reduce costs.


A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company.[1] Biosimilars are officially approved versions of original “innovator” products and can be manufactured when the original product’s patentexpires.[2] Reference to the innovator product is an integral component of the approval.
Unlike with generic drugs of the more common small-molecule type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle.[3] Follow-on manufacturers do not have access to the originator’s molecular clone and original cell bank, to the exact fermentation and purification process, or to the active drug substance, but they have access to the commercialized innovator product. Overall, it is harder to ascertain fungibility between generics and innovators among biologics than it is among totally synthesized and semisynthesized drugs. That is why the name “biosimilar” was coined to differentiate them from small-molecule generics. A simple analogy, often used to explain the difference, is to compare wine with soda pop. It is harder to say objectively that two bottles of wine from two wineries are “sufficiently interchangeable,” because of differences in yeast strain, weather, and year of grape harvest, than it is to say that two bottles of soda pop of the same flavor coming from two bottling plants are “sufficiently interchangeable” because they contain the same flavoring powder.
Drug-related authorities such as the EU’s European Medicines Agency (EMA), the US’s Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada hold their own guidance on requirements for demonstration of the similar nature of two biological products in terms of safety and efficacy. According to them, analytical studies demonstrate that the biological product is highly similar to the reference product, despite minor differences in clinically inactive components, animal studies (including the assessment of toxicity), and a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics). They are sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed and is intended to be used and for which licensure is sought for the biological product.
In case of a monoclonal antibody-containing medicinal product, such as Remsima, extensive physicochemical and biological characterization for it and its reference product Remicade was conducted to demonstrate their highly-similar properties. Therefore, EMA has granted a marketing authorisation for only a few biosimilars since 2006, including a monoclonal antibody, that was recently approved. Meanwhile, on March 6, 2015, the FDA approved the United States’s first biosimilar product, the biosimilar of filgrastim called filgrastim-sndz (trade name Zarxio) by Sandoz.
Contents
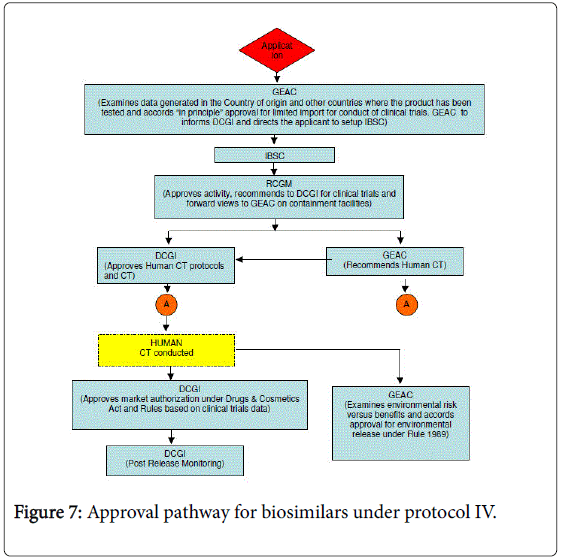
Approval processes
The European regulatory authorities led with a specially adapted approval procedure to authorize subsequent versions of previously approved biologics, termed “similar biological medicinal products”, or biosimilars. This procedure is based on a thorough demonstration of “comparability” of the “similar” product to an existing approved product.[4] In the United States, the Food and Drug Administration (FDA) held that new legislation was required to enable them to approve biosimilars to those biologics originally approved through the PHS Act pathway.[5] Additional Congressional hearings have been held.[6] On March 17, 2009, the Pathway for Biosimilars Act was introduced in the House.[2] See the Library of Congress website and search H.R. 1548 in 111th Congress Session. Since 2004 the FDA has held a series of public meetings on biosimilars.[7][8]
The FDA gained the authority to approve biosimilars (including interchangeables that are substitutable with their reference product) as part of the Patient Protection and Affordable Care Act signed by President Obama on March 23, 2010.
The FDA has previously approved biologic products using comparability, for example, Omnitrope in May 2006, but this like Enoxaparin was also to a reference product, Genotropin, originally approved as a biologic drug under the FD&C Act.[9]
On March 6, 2015, Zarxio obtained the first approval of FDA.[10] Sandoz’s Zarxio is biosimilar to Amgen’s Neupogen (filgrastim), which was originally licensed in 1991. This is the first product to be passed under the Biologics Price Competition and Innovation Act of 2009 (BPCI Act), which was passed as part of the Affordable Healthcare Act. But Zarxio was approved as a biosimilar, not as an interchangeable product, the FDA notes. And under the BPCI Act, only a biologic that has been approved as an “interchangeable” may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product. The FDA said its approval of Zarxio is based on review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
Background
Cloning of human genetic material and development of in vitro biological production systems has allowed the production of virtually any recombinant DNA based biological substance for eventual development of a drug. Monoclonal antibody technology combined with recombinant DNA technology has paved the way for tailor-made and targeted medicines. Gene-and cell-based therapies are emerging as new approaches.
Recombinant therapeutic proteins are of a complex nature (composed of a long chain of amino acids, modified amino acids, derivatized by sugar moieties, folded by complex mechanisms). These proteins are made in living cells (bacteria, yeast, animal or human cell lines). The ultimate characteristics of a drug containing a recombinant therapeutic protein are to a large part determined by the process through which they are produced: choice of the cell type, development of the genetically modified cell for production, production process, purification process, formulation of the therapeutic protein into a drug.
After the expiry of the patent of approved recombinant drugs (e.g., insulin, human growth hormone, interferons, erythropoietin, monoclonal antibodies and more) any other biotech company can develop and market these biologics (thus called biosimilars). Every biological (or biopharmaceutical products) displays a certain degree of variability, even between different batches of the same product, which is due to the inherent variability of the biological expression system and the manufacturing process.[11] Any kind of reference product has undergone numerous changes in its manufacturing processes, and such changes in the manufacturing process (ranging from a change in the supplier of cell culture media to new purification methods or new manufacturing sites) was substantiated with appropriate data and was approved by the EMA. In contrast, it is mandatory for biosimilars to take a both non-clinical and clinical test that the most sensitive clinical models are asked to show to enable detection of differences between the two products in terms of human pharmacokinetics (PK) and pharmacodynamics (PD), efficacy, safety, and immunogenicity.
The current concept of development of biosimilar mAbs follows the principle that an extensive state of the art physicochemical, analytical and functional comparison of the molecules is complemented by comparative non-clinical and clinical data that establish equivalent efficacy and safety in a clinical “model” indication that is most sensitive to detect any minor differences (if these exist) between biosimilar and its reference mAb also at the clinical level.
The European Medicines Agency (EMA) has recognized this fact, which has resulted in the establishment of the term “biosimilar” in recognition that, whilst biosimilar products are similar to the original product, they are not exactly the same.[12] Every biological displays a certain degree of variability. However, provided that structure and function(s), pharmacokinetic profiles and pharmacodynamic effect(s) and/or efficacy can be shown to be comparable for the biosimilar and the reference product, those adverse drug reactions which are related to exaggerated pharmacological effects can also be expected at similar frequencies.
Originally the complexity of biological molecules led to requests for substantial efficacy and safety data for a biosimilar approval. This has been progressively replaced with a greater dependence on assays, from quality through to clinical, that show assay sensitivity sufficient to detect any significant difference in dose.[13] However, the safe application of biologics depends on an informed and appropriate use by healthcare professionals and patients. Introduction of biosimilars also requires a specifically designed pharmacovigilance plan. It is difficult and costly to recreate biologics because the complex proteins are derived from living organisms that are genetically modified. In contrast, small molecule drugs made up of a chemically based compound can be easily replicated and are considerably less expensive to reproduce. In order to be released to the public, biosimilars must be shown to be as close to identical to the parent innovator biologic product based on data compiled through clinical, animal, analytical studies and conformational status.[14][15]
Generally, once a drug is released in the market by FDA, it has to be re-evaluated for its safety and efficacy once every six months for the first and second years. Afterward, re-evaluations are conducted yearly, and the result of the assessment should be reported to authorities such as FDA. Biosimilars are required to undergo pharmacovigilance (PVG) regulations as its reference product. Thus biosimilars approved by EMEA (European Medicines Agency) are required to submit a risk management plan (RMP) along with the marketing application and have to provide regular safety update reports after the product is in the market. The RMP includes the safety profile of the drug and proposes the prospective pharmacovigilance studies.
Several PK studies, such as studies conducted by Committee for Medicinal Products for Human Use (CHMP), have been conducted under various ranges of conditions; Antibodies from an originator’s product versus antibodies from an biosimilar; combination therapy and monotherapy; various diseases, etc. on the purpose to verify comparability in pharmacokinetics of the biosimilar with the reference medicinal product in a sufficiently sensitive and homogeneous population. Importantly, provided that structure and function(s), pharmacokinetic profiles and pharmacodynamic effect(s) and/or efficacy can be shown to be comparable for the biosimilar and the reference product, those adverse drug reactions which are related to exaggerated pharmacological effects can also be expected at similar frequencies.
European approvals of biosimilars
It has approved (in Jan 2016) Benepali a biosimilar to Enbrel (etanercept).[16]
United States of America
BPCI Act
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was originally sponsored and introduced on June 26, 2007, by Senator Edward Kennedy (D-MA). It was formally passed under the Patient Protection and Affordable Care Act (PPAC Act), signed into law by President Barack Obama on March 23, 2010. The BPCI Act was an amendment to the Public Health Service Act (PHS Act) to create an abbreviated approval pathway for biological products that are demonstrated to be highly similar (biosimilar) to a Food and Drug Administration (FDA) approved biological product. The BPCI Act is similar, conceptually, to the Drug Price Competition and Patent Term Restoration Act of 1984 (also referred to as the “Hatch-Waxman Act”) which created biological drug approval through the Federal Food, Drug, and Cosmetic Act (FFD&C Act). The BPCI Act aligns with the FDA’s longstanding policy of permitting appropriate reliance on what is already known about a drug, thereby saving time and resources and avoiding unnecessary duplication of human or animal testing. The FDA has released a total of four draft guidelines related to biosimilar or follow-on biologics development. Upon the release of the first three guidance documents the FDA held a public hearing on May 11, 2012.[17]
Data exclusivity
Data exclusivity is an important piece of the amendment in the Patient Protection and Affordable Care Act for biosimilars. It is the period of time between FDA approval and an abbreviated filing for a biosimilar on the original producer’s data. Data exclusivity is designed to preserve innovation and recognize the long, costly, and risky process involved while the innovator waits to gain FDA approval. The time allowed for data exclusivity is critical for the future of biologics. A number of provisions for data exclusivity in recent legislative proposals ranged up to 14 years, however, the passing of the PPAC Act guarantees a 12-year time period from the time of FDA approval.[18] This is supposed to compensate for perceived shortcomings in patent protection for biologics. Data exclusivity extends from the date of product approval, and this protection period runs concurrently with any remaining patent term protection for the biologic. That is to say, data exclusivity provides additional protection to the innovator when the remaining patent length is shorter than the data exclusivity period at the time of approval (which can occur due to lengthy pre-clinical and clinical research required to obtain FDA approval), or to the extent that the patent term is circumvented by a biosimilar prior to its expiry.
US approved biosimilars
| Date of Biosimilar FDA Approval | Biosimilar Product | Original Product |
|---|---|---|
| March 6, 2015[19] | filgrastim-sndz/Zarxio | filgrastim/Neupogen |
| April 5, 2016[20] | infliximab-dyyb/Inflectra | infliximab/Remicade |
| August 30, 2016[21] | etanercept-szzs/Erelzi | etanercept/Enbrel |
| September 23, 2016[22] | adalimumab-atto/Amjevita | adalimumab/Humira |
| April 21, 2017[23] | infliximab-abda/Renflexis | infliximab/Remicade |
| August 25, 2017[24] | adalimumab-adbm/Cyltezo | adalimumab/Humira |
| September 14, 2017[25] | bevacizumab-awwb/Mvasi | bevacizumab/Avastin |
| December 1, 2017[26] | trastuzumab-dkst/Ogivri | trastuzumab/Herceptin |
| December 13, 2017[27] | infliximab-qbtx/Ixifi | infliximab/Remicade |
| May 15, 2018[28] | epoetin alfa-epbx/Retacrit | epoetin alfa/Procrit |
| June 4, 2018[29] | pegfilgrastim-jmdb/Fulphila | pegfilgrastim/Neulasta |
Nomenclature
The World Health Organization (WHO) and the FDA have been working for years on the nonproprietary naming of biosimilars. In August 2015, the FDA published a draft guideline on the topic.[30] In brief, the guideline calls for the assignment of a four character alphabetic suffix to the nonproprietary name of the original product to distinguish between innovator drugs and their biosimilars.[31] The WHO INN system calls this suffix a biologic modifier.[32]
Market implications
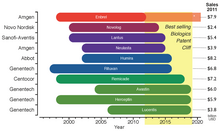
The 2012–2019 patent cliff.[33]Period of market exclusivity up to date of patent expiration for the ten top-selling biologics for 2011. *Enbrel has been granted approval in 2011 for a patent filed in 1995, extending its patent life further 17 years.
The legal requirements of approval pathways, together with the costly manufacturing processes, escalates the developing costs for biosimilars that could be between $75–$250 million per molecule.[33] This market entry barrier affects not only the companies willing to produce them but could also delay availability of inexpensive alternatives for public healthcare institutions that subsidize treatment for their patients. Even though the biosimilars market is rising, the price drop for biological drugs at risk of patent expiration will not be as great as for other generic drugs; in fact it has been estimated that the price for biosimilar products will be 65%-85% of their originators.[33] Biosimilars are drawing market’s attention since there is an upcoming patent cliff, which will put nearly 36% of the $140 billion market for biologic drugs at risk (as of 2011), this considering only the top 10 selling products.[33]
The global biosimilars market was $1.3 billion in 2013 and is expected to reach $35 billion by 2020 driven by the patent expiration of additional ten blockbuster biologic drugs.[34]
References
- Jump up^ Blanchard, A., Helene D’Iorio and Robert Ford. “What you need to know to succeed: Key trends in Canada’s biotech industry ” Insights, spring 2010
- ^ Jump up to:a b Nick, C (2012). “The US Biosimilars Act: Challenges Facing Regulatory Approval”. Pharm Med. 26 (3): 145–152. doi:10.1007/bf03262388.
- Jump up^ Lamanna, William C.; Holzmann, Johann; Cohen, Hillel P.; Guo, Xinghua; Schweigler, Monika; Stangler, Thomas; Seidl, Andreas; Schiestl, Martin (2018-01-10). “Maintaining consistent quality and clinical performance of biopharmaceuticals”. Expert Opinion on Biological Therapy: 1–11. doi:10.1080/14712598.2018.1421169. ISSN 1744-7682. PMID 29285958.
- Jump up^ “EMEA Guideline on Similar Biological Medicinal Products, CHMP/437/04 London, 30 October 2005”.
- Jump up^ “US Senate Committee on the Judiciary, Testimony of Dr. Lester Crawford, Acting Commissioner, FDA June 23, 2004”.
- Jump up^ Hearing: Assessing the Impact of a Safe and Equitable Biosimilar Policy in the United States. Subcommittee on Health Wednesday, May 2, 2007 Archived September 22, 2007, at the Wayback Machine.
- Jump up^ “FDA page on “Follow-On Protein Products: Regulatory and Scientific Issues Related to Developing““.
- Jump up^ “FDA page on “Approval Pathway for Biosimilar and Interchangeable Biological Products Public Meeting““.
- Jump up^ “FDA Response to three Citizen Petitions against biosimilars”(PDF).
- Jump up^ “FDA page on “FDA approves first biosimilar product Zarxio““.
- Jump up^ Martina Weise (October 8, 2014). “Biosimilars: the science of extrapolation”. Blood. 124 (22): 3191–6. doi:10.1182/blood-2014-06-583617. PMID 25298038.
- Jump up^ EMEA guideline on similar biological medicinal productsArchived June 30, 2007, at the Wayback Machine.
- Jump up^ Warren, JB (2013). “Generics, chemisimilars and biosimilars: is clinical testing fit for purpose?”. Br J Clin Pharmacol. 75 (1): 7–14. doi:10.1111/j.1365-2125.2012.04323.x. PMC 3555041. PMID 22574725.
- Jump up^ Wang, X. (June 1, 2014). “Higher-Order Structure Comparability: Case Studies of Biosimilar Monoclonal Antibodies”. BioProcess International. 12 (6): 32–37.
- Jump up^ Declerck PJ (February 2013). “Biosimilar monoclonal antibodies: a science-based regulatory challenge”. Expert Opin Biol Ther. 13 (2): 153–6. doi:10.1517/14712598.2012.758710. PMID 23286777.
- Jump up^ “Pfizer expects major biosimilar impact… but not immediately”.
- Jump up^ Epstein, MS; Ehrenpreis, ED; Kulkarni, PM; FDA-Related Matters Committee of the American College of, Gastroenterology (December 2014). “Biosimilars: the need, the challenge, the future: the FDA perspective” (PDF). The American Journal of Gastroenterology. 109 (12): 1856–9. doi:10.1038/ajg.2014.151. PMID 24957160.
- Jump up^ “42 U.S. Code § 262 – Regulation of biological products”. LII / Legal Information Institute.
- Jump up^http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm436648.htm
- Jump up^ “Press Announcements – FDA approves Inflectra, a biosimilar to Remicade”. www.fda.gov.
- Jump up^ “Press Announcements – FDA approves Erelzi, a biosimilar to Enbrel”. www.fda.gov.
- Jump up^ “Press Announcements – FDA approves Amjevita, a biosimilar to Humira”. www.fda.gov.
- Jump up^https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761054lbl.pdf
- Jump up^ “Drugs@FDA: FDA Approved Drug Products”. www.accessdata.fda.gov. Retrieved 2017-08-28.
- Jump up^ Commissioner, Office of the. “Press Announcements – FDA approves first biosimilar for the treatment of cancer”. www.fda.gov.
- Jump up^ Commissioner, Office of the. “Press Announcements – FDA approves first biosimilar for the treatment of certain breast and stomach cancers”. www.fda.gov.
- Jump up^ “FDA Approves New Pfizer Biosimilar – Pfizer: One of the world’s premier biopharmaceutical companies”. www.pfizer.com.
- Jump up^ Commissioner, Office of the. “Press Announcements – FDA approves first epoetin alfa biosimilar for the treatment of anemia”. www.fda.gov.
- Jump up^ Commissioner, Office of the. “Press Announcements – FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment”. www.fda.gov.
- Jump up^ “Naming and Biological Products | FDA Voice”. blogs.fda.gov. Retrieved 2015-08-29.
- Jump up^ “Federal Register | Nonproprietary Naming of Biological Products; Draft Guidance for Industry; Availability”. www.federalregister.gov. Retrieved 2015-08-29.
- Jump up^ World Health Organization, INN and Biologicals, retrieved 2016-08-05.
- ^ Jump up to:a b c d Calo-Fernández B, Martínez-Hurtado J (December 2012). “Biosimilars: Company Strategies to Capture Value from the Biologics Market”. Pharmaceuticals. 5 (12): 1393–1408. doi:10.3390/ph5121393. PMC 3816668. PMID 24281342.
- Jump up^ “Biosimilars and Follow-on-Biologics Market to Hit $35 Billion Globally by 2020”. Pharmaceutical Technology. 28 August 2015.
Further reading
- Udpa, Natasha; Million, Ryan P. (18 December 2015). “Monoclonal antibody biosimilars”. Nature Reviews Drug Discovery. 15 (1): 13–4. doi:10.1038/nrd.2015.12. PMID 26678619. Retrieved 22 December 2015.

- Jelkmann, Wolfgang (2010). “Biosimilar epoetins and other “follow-on” biologics: update on the European experiences”. American Journal of Hematology. 85 (10): 771–80. doi:10.1002/ajh.21805. PMID 20706990.
- “New guide on biosimilar medicines for healthcare professionals”. Prepared jointly by the European Medicines Agency and the European Commission. Retrieved 10 May 2017.
- https://www.pbs.org/newshour/bb/whats-keeping-generic-version-biologic-drugs-u-s-market/
////////////
11,630 total views, 1 views today