Itraconazole has been found to possess potent antiangiogenic activity, exhibiting promising antitumor activity in several human clinical studies. The wider use of itraconazole in the treatment of cancer, however, has been limited by its potent inhibition of the drug metabolizing enzyme cytochrome P450 3A4 (CYP3A4). In an effort to eliminate the CYP3A4 inhibition while retaining its antiangiogenic activity, we designed and synthesized a series of derivatives in which the 1,2,4-triazole ring is replaced with various azoles and nonazoles. Among these analogues, 15n with tetrazole in place of 1,2,4-triazole exhibited optimal inhibition of human umbilical vein endothelial cell proliferation with an IC50 of 73 nM without a significant effect on CYP3A4 (EC50 > 20 μM). Similar to itraconazole, 15n induced Niemann-Pick C phenotype (NPC phenotype) and blocked AMPK/mechanistic target of rapamycin signaling. These results suggest that 15n is a promising angiogenesis inhibitor that can be used in combination with most other known anticancer drugs.
Novel Tetrazole-Containing Analogues of Itraconazole as Potent Antiangiogenic Agents with Reduced Cytochrome P450 3A4 Inhibition
†Department of Pharmacology and Molecular Sciences and ‡Department of Oncology, Johns Hopkins School of Medicine, Baltimore, Maryland 21205, United States
J. Med. Chem., 2018, 61 (24), pp 11158–11168
DOI: 10.1021/acs.jmedchem.8b01252
Publication Date (Web): November 27, 2018
Copyright © 2018 American Chemical Society
https://pubs.acs.org/doi/10.1021/acs.jmedchem.8b01252
■ ASSOCIATED CONTENT *S Supporting Information The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01252. Molecular formula strings (CSV) Detail of synthesis procedures; kinetic curve of CYP3A4 enzyme activities; philipin staining of compound 15c, 15g; competition assay of itraconazole photoaffinity probe; and NMR and HPLC chart of representative compounds (PDF)
■ AUTHOR INFORMATION Corresponding Author *E-mail: joliu@jhu.edu. Phone 410-955-4619. Fax 410-955- 4520. ORCID Wei Q. Shi: 0000-0001-5453-1753 Jun O. Liu: 0000-0003-3842-9841 Author Contributions § Y.L. and K.K.P. contributed equally to this work. Notes The authors declare no competing financial interest.
■ ACKNOWLEDGMENTS This work was supported by the National Cancer Institutes (grant R01CA184103) and the Flight Attendant Medical Research Institute
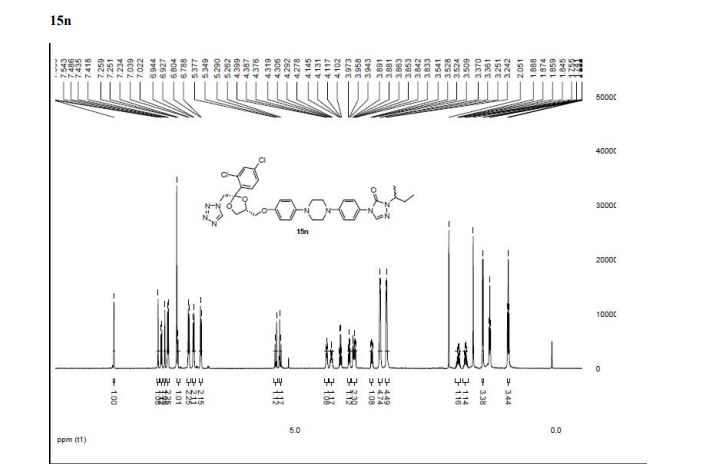
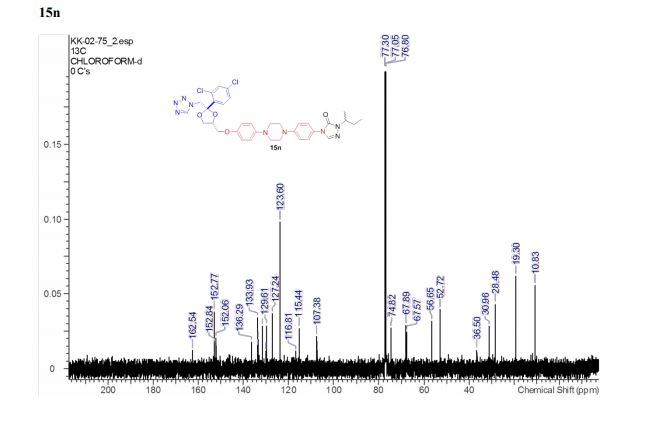
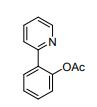
4-(4-(4-(4-(((2S,4R)-2-((1H-Tetrazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazin-1-yl)phenyl)- 1-sec-butyl-1H-1,2,4-triazol-5(4H)-one (15n).
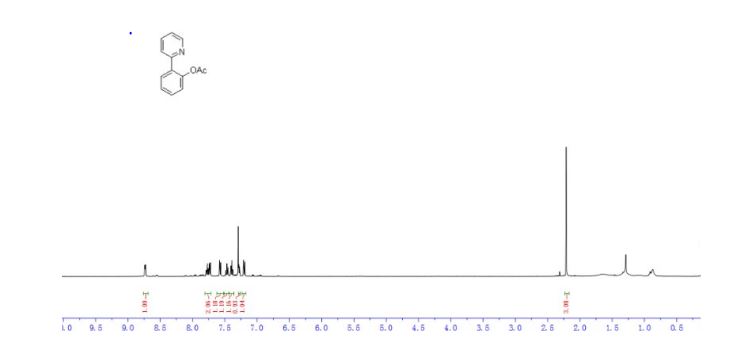
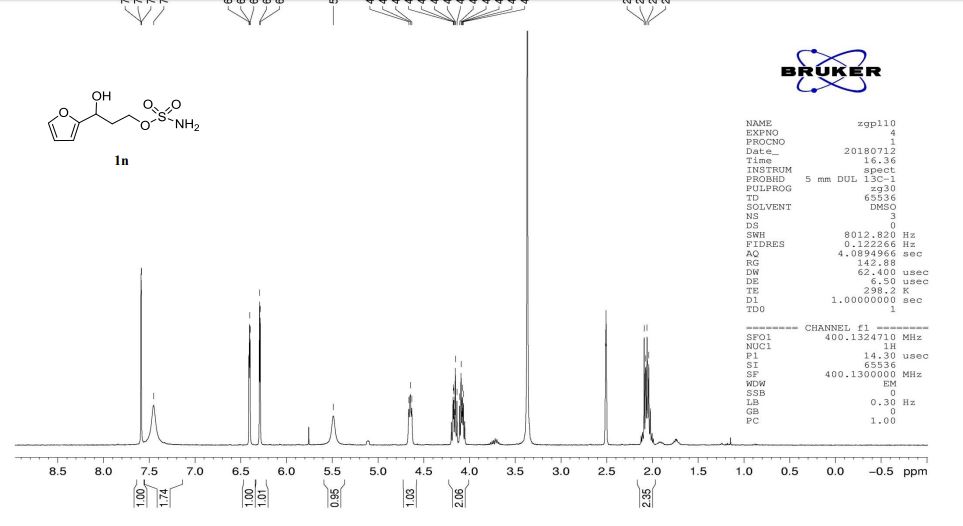
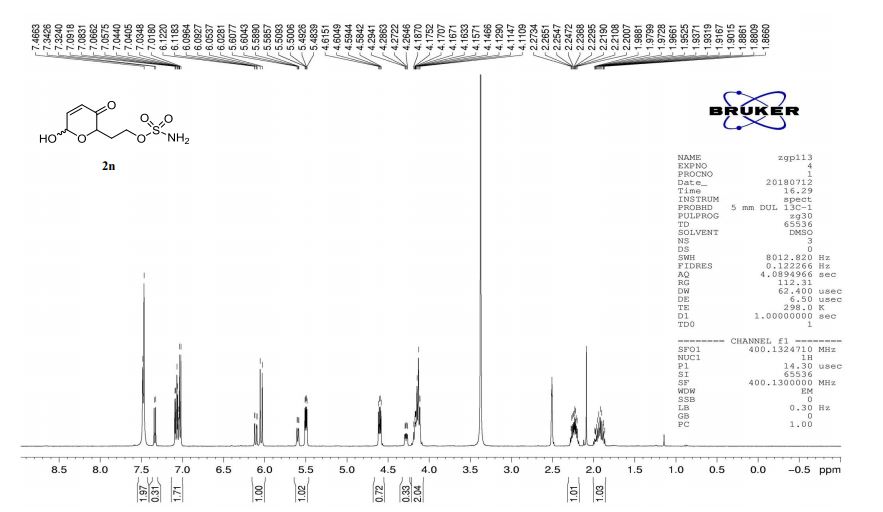
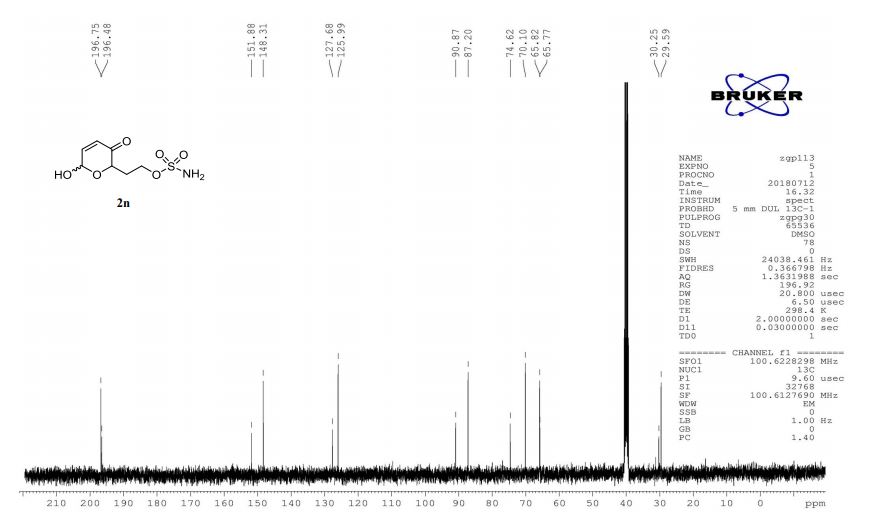
1 H NMR (500 MHz, CDCl3, δH): 8.46 (s, 1H), 7.61 (s, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.48 (d, J = 2.0 Hz, 1H), 7.43 (d, J = 9 Hz, 2H), 7.24 (dd, J = 8.5, 2.0 Hz, 1H), 7.03 (d, J = 9.0 Hz, 2H), 6.81 (d, J = 9.0 Hz, 2H), 5.36 (d, J = 14.0 Hz, 1H), 5.27 (d, J = 14.0 Hz, 1H), 4.38 (t, J = 5.0 Hz, 1H), 4.31−4.27 (m, 1H), 3.95 (dd, J = 8.5, 6.5 Hz, 1H), 3.88−3.83 (m, 2H), 3.53 (dd, J = 9.5, 6.5 Hz, 1H), 3.38 (br s, 4H), 3.26 (br s, 4H), 1.89−1.83 (m, 1H), 1.74−1.69 (m, 1H), 1.39 (d, J = 7.0 Hz, 3H), 0.90 (t, J = 7.5 Hz, 3H).
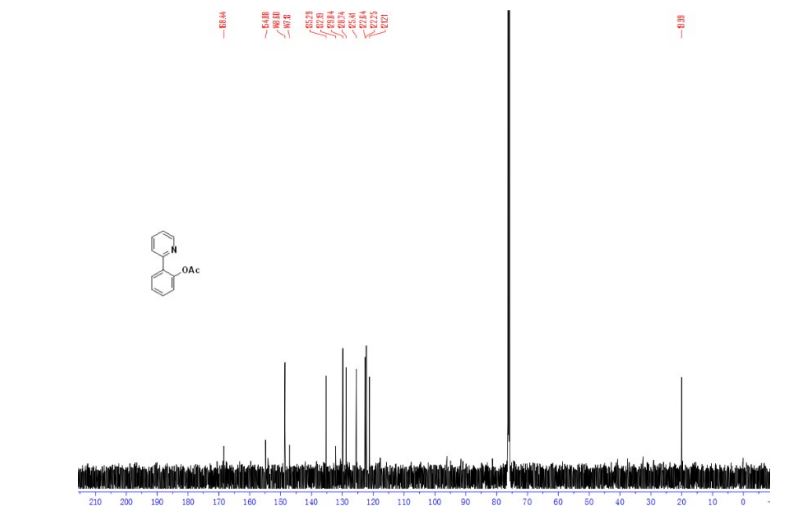
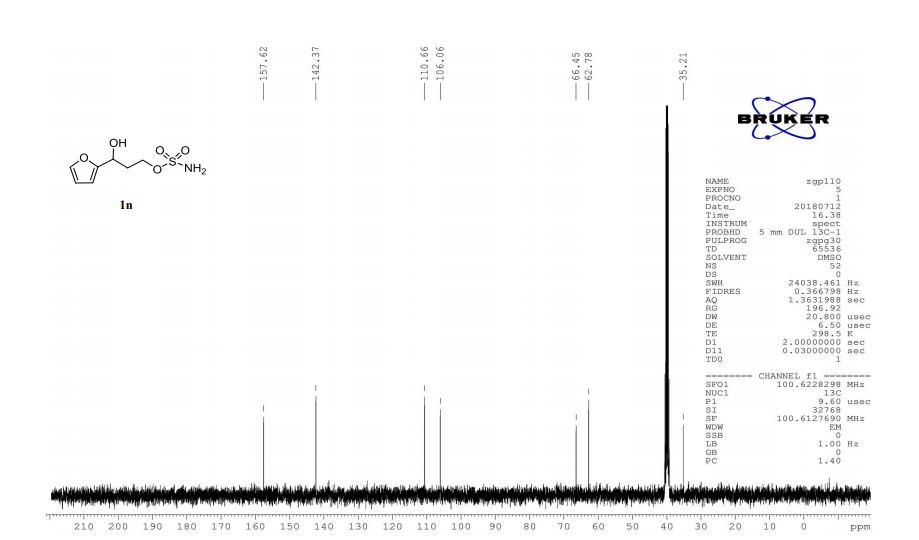
13C NMR (125 MHz, CDCl3, δC): 162.5, 152.8, 152.7, 152.0, 136.3, 133.9, 133.3, 131.5, 130.1, 129.6, 127.2, 123.6, 116.8, 115.4, 107.4, 74.8, 67.9, 67.6, 56.6, 52.7, 36.5, 31.0, 28.5, 19.2, 10.8.
HRMS (ESI) calcd for C34H37Cl2N9O4, 706.2424; found, 706.2425.
HPLC purity: 95.9%, tR = 10.5 min

Kalyan Kumar Pasunooti,
kalyan kumar <kalyandrf@gmail.com>
|
|
|
Dr. Kalyan Kumar Pasunooti pursued his PhD degree from Nanyang Technological University (NTU) (www.ntu.edu.sg), Singapore (2007 – 2011) in the field of Medicinal, Peptide & Protein chemistry. His graduate research work is focused on “Synthesis of bioactive amino acid building blocks and their applications towards the peptides and glycopeptides.” His have total 16 years of academic and industry experience with major multinationals companies & academic institutions and have worked with many collaborative professors around the globe. He authored with more than 28 international peer-reviewed high impact publications such as PNAS, Wily (Angew Chemie), RSC (Chem Comm and Org Biomol Chem), most of American Chemical Society journals (Journal of American Chemical Society, Org. Lett., ACS Chem Bio, J Comb Chem and Bioconugate Chem) and Elsevier (Tetrahedron Letters) journals which are featured many times in Chem. Eng. News and other journals. He holds American patent while work with Johns Hopkins-School of Medicine, USA and this molecule in phase II clinical trials for treating cancer.
Prior to his graduate studies, he spent 5 years as a research scientist in reputable research organizations namely GVK Bio, India (www.gvkbio.com) (2006-2007) and Dr. Reddy’s Laboratories Ltd (www.drreddys.com) (2003-2006) in India. After his PhD graduation, he worked for world leading research institutes such as Johns Hopkins-School of Medicine, USA (www.hopkinsmedicne.org) (2012-2013), Nanyang Technological University-NTU, Singapore) (www.ntu.edu.sg) (2013 – 2017) and Singapore MIT Alliance for research & Technology-SMART (www.smart.mit.edu) (2017–2018). His research interests focused on development of next generation biologically relevant peptide & protein therapeutics using their newly discovered methodologies for biomedical applications.
He has excellent skills in designing synthesis, purification and characterization of complex peptide and small molecules for medicinal chemistry applications. He gained extensive experience in Medicinal, Carbohydrate, Peptide & Protein and nucleotide & nucleoside Chemistry and familiar with modern methods and experienced in designing & executing synthesis for various bioactive peptide and small molecule inhibitors. He well versed in synthesis and characterization of complex organic molecules and with the analytical data interpretation.
Dr. Kalyan Kumar Pasunooti
Research Scientist at Singapore-MIT Alliance for Research & Technology Centre
Singapore’
Accomplished Peptide, Protein and Medicinal chemist with 16 years of academic and industrialexperience in the field of drug discovery and development. Specializations: Peptide & Protein Chemistry,Medicinal Chemistry (Drug Discovery and Development) and Chemical Biology.
READ
ANTHONY MELVIN CRASTO
https://newdrugapprovals.org/

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on Linkedin

Join me on Facebook  FACEBOOK
FACEBOOK
Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////




















 READ
READ
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..